Abstract
Specimens of Foleyellides were collected from the body cavity of frogs in different regions of Mexico; Lithobates brownorum from Yucatán, Quintana Roo and Campeche; L. megapoda from Jalisco and Rhinella marina, from Guerrero. Foleyellides calakmulesis n. sp. is described based on specimens found parasitizing L. brownorum. The new species is distinguished from the other members of the genus by the combination of the following male characters: four pairs of caudal papillae different in size and the presence of a preanal plaque. Partial DNA sequences of the mitochondrial Cytochrome Oxidase C, subunit I of the four known Mexican species of Foleyellides and two potentially new species collected in this study were generated and compared, validating the erection of the new species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ochoterena and Caballero (1932) described Chandlerella striatus Ochoterena and Caballero, 1932 parasitizing the body cavity of Lithobates montezumae from central Mexico. A few years later, Caballero (1935) reassigned C. striatus as the type species of the new genus Foleyellides Caballero, 1935, which some authors considered synonym of Foleyella Seurat, 1917 (Anderson & Bain, 1976; López-Neyra, 1956; Witenerg & Gerichter, 1994; Yamaguti, 1961), or Waltonella Schacher, 1974 (Bain & Prod’hon, 1974) (for a detailed review see Romero-Mayén & León-Règagnon, 2016).
Esslinger (1986) re-examined specimens of Foleyellides striatus (Ochoterena and Caballero, 1932) Caballero, 1935 and reinstated Foleyellides as a valid taxon, currently including 11 species: Foleyellides americana (Walton, 1929); F. brachyoptera (Wehr and Causey, 1939); F. confusa Schmidt and Kuntz, 1969; F. dolichoptera (Wehr and Causey, 1939); F. duboisi (Gedoelst, 1916); F. flexicauda (Schacher and Crans, 1973); F. malayensis (Petit and Yen, 1979); F. mayenae Romero-Mayén and León-Règagnon, 2016; F. ranae (Walton, 1929); F. rhinellae García-Prieto, Ruiz-Torres, Osorio-Sarabia and Merlo-Serna, 2014; and the type species, F. striatus. Eight of these species are distributed in North America (five in the United States of America and three in Mexico); two more in Asia, and one in Africa.
Mitochondrial DNA sequences, in particular partial COI sequences, known as barcodes, have shown to be useful to differentiate species of nematodes (Lima-Monteiro et al., 2018; Powers et al., 2018; Siddall et al., 2012), given the morphological conservatism in some groups and high phenotypic plasticity in others (Nadler, 2002; Powers et al., 2011). Nevertheless, the only barcodes representing Foleyellides available in GenBank belong to F. mayenae. The aim of this study is to describe a new species of Foleyellides from southeast Mexico based on morphological and molecular evidence, as well as to ameliorate the lack of molecular information for this group available in public repositories, since partial sequences of the mitochondrial COI gene were generated for the four known Mexican species and two potentially new species collected in this study.
Materials and methods
Specimens collection
Specimens of Lithobates brownorum were collected in Yucatán, Quintana Roo and Campeche, Mexico, during June and July 2016 (table 1). Specimens were collected under the scientific collection permits FAUT0056 issued to VLR and SGPA/DGVS/ 02798/16 to AOF by Secretaría del Medio Ambiente y Recursos Naturales (SEMARNAT). Amphibians were captured using dip nets and euthanized by an overdose of sodium pentobarbital, dissected and examined under stereomicroscope; worms were placed in saline solution (0.65%) for 4–8 min and examined in vivo for distinctive morphological traits. For morphological study, specimens were fixed in hot ethanol (96%) and preserved in ethanol (70%). For molecular analyses, worms were fixed and preserved in ethanol (100%). Nematodes previously collected and identified as F. striatus of L. megapoda from Jalisco (July 2012) and F. rhinellae of Rhinella marina from Guerrero (August 2010) were also processed for molecular analyses (table 1).
Morphological analyses
Specimens were cleared with glycerine for 24 h and mounted between coverslips; measurements are given in millimetres (unless otherwise indicated), with minimum and maximum, mean and standard deviation in parentheses. Drawings were made using a camera lucida attached to a microscope. Helminth specimens were deposited in the Colección Nacional de Helmintos (CNHE), Instituto de Biología, Universidad Nacional Autónoma de México, Mexico City. Host specimens were desposited in the Colección Nacional de Anfibios y Reptiles (CNAR). For scanning electron microscopy (SEM), specimens were dehydrated through a graded ethanol series, critical point dried with K850 Critical Point Drier (Emitech, Ashford, England), sputter-coated with gold with Q150R Modular Coating System (Quo´Rum, Ashford, England), and examined with a Hitachi SU1510 SEM (Hitachi, Tokyo, Japan) at the Laboratorio Nacional de la Biodiversidad (LANABIO), Instituto de Biología, Universidad Nacional Autónoma de México.
Molecular analyses
For molecular analyses, total DNA was extracted using the Jena Bioscience kit, following the protocol provided by the company (Jena Bioscience, Jena, Germany). Amplification and sequencing of partial DNA sequences of the mitochondrial Cytochrome Oxidase C subunit I (COI) locus were carried out using a cocktail of six primers: forward (C_NemF1t1: NemF1_t1+NemF2_t1+NemF3_t1), reverse (C_NemR1_t1: NemR1_t1+NemR2_t1 +NemR3_t1) (Prosser et al., 2013). Thermal cycling conditions for amplification reactions were 94 C for 1 min, five cycles at 94 C for 45 s, 45 C for 40 s, 72 C for 1 min, followed by 35 cycles at 94 C for 40 s, 51 C for 40 s, 72 C for 1 min and a final extension at 72 C for 5 min. Sequencing reactions were accomplished using an ABI 3730xl Genetic Analyzer (Thermo Fisher Scientific, Waltham, Massachusetts, USA) at the LANABIO.
Sequences were edited and assembled using the program Geneious 5.1.7 (Biomatters Ltd. Auckland, New Zealand). For phylogenetic analyses, sequences of Foleyellides mayenae and other species included in Waltonellinae were downloaded from GenBank (table 1), Icosiella neglecta, Oswaldofilaria petersi, Oswaldofilaria chabaudi and Onchocerca volvulus were used as outgroup (table 1). DNA sequences were aligned using MAFFT (Katoh & Standley, 2013), with the default parameters. Uncorrected P distances were obtained in MEGA-X (Kumar et al., 2018).
The phylogenetic analysis was performed through Bayesian inference (BI), using Markov Chain Monte Carlo (MCMC) in Mr. Bayes V 3.1.2 (Ronquist et al., 2012). The appropriate model of evolution (GTR+1+Γ) was determined with jModeltest 0.1.1 (Posada, 2008). The chains run for 1,500,000 generations, sampling trees every 1,000 generations; the first 25% of the sampled trees were discarded according to Tracer V 1.5 (htt://beast.bio.ed.ac.uk/ tracer); consensus topology and posterior probability values were calculated from the remaining 75% of the trees.
Results
Description
Onchocercidae Leiper, 1911
Foleyellides Caballero, 1935
Foleyellides calakmulensis n. sp.
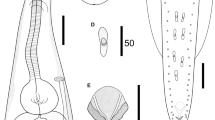
Foleyellides calakmulensis n. sp. Line drawing; male, ventral view of posterior end (a); male, lateral view of posterior end (b); apical view showing four pairs of papillae and parastomal structures (c); female, lateral view of anterior region showing position of vulva (d); male, ventral view of anterior end (e).
SEM of a male of Foleyellides calakmulensis n. sp. Lateral view of anterior region (a); apical view of anterior region showing four pairs of papillae (b); apical view showing parastomal structures (c); male, ventral view of caudal region, showing the distribution and size of papillae, preanal plaque and spicules (d).
Male (based on 18 mature specimens): body length 12.57–20.26 (15.55 ± 1.84), wide at mid-hind body 0.21–0.40 (0.31±0.05). Maximum width at nerve ring level 0.15–0.20 (0.17 ± 0.016); at muscular/glandular esophagus junction 0.15–0.22 (0.18 ± 0.019) and at esophagus-intestinal junction 0.20–0.40 (0.30 ± 0.05). Cephalic extremity with one pair of cuticularized parastomal structures. Four pairs of cephalic papillae. Cephalic plate 30–60 (47 ± 6) µm long, 13–26 (20 ± 2) µm wide. Esophagus total length 1.301–2.12 (1.67 ± 0.22), muscular portion 0.17–0.40 (0.27 ± 0.06) long, 0.02–0.04 (0.03 ± 0.007) wide, glandular portion 1.11–1.80 (1.44 ± 1.16) long, 0.06–0.11 (0.08 ± 0.01) wide; ratio length glandular to muscular 1:0.123–0.256 (0.188 ± 0.03). Nerve ring 0.158–0.3 (0.22 ± 0.03) from anterior end. Tail length 0.08–0.17 (0.11 ± 0.03); dorsoventral thickness of body at level of anus 0.05–0.09 (0.07 ± 0.009). Four pairs of large and sessile caudal papillae; 1 pre-anal pair, 3 post-anal pairs; posterior pair 0.01–0.04 (0.02 ± 0.008) from tip of tail. Preanal cuticularized ventral plaque, well developed, thin and large, anterior to caudal papillae. Spicules unequal in form and size, right 125.3–247 (186.3 ± 32) µm long by 2.6–9 (4.9 ± 1.9) µm wide at base; left 265.3–375.3 (311.6 ± 32.2) µm long by 2.5–7.5 (5 ± 1.2) µm wide at base. Tail 0.08-0.17 (0.11 ± 0.03) long. Area rugosa as well as lateral and caudal alae well developed.
Female (based on 12 gravid specimens): body length 19.14–49.13 (31.57 ± 8.58); width at mid-hind body 0.27–0.77 (0.49 ± 0.15). Maximum width at nerve ring level 0.163–0.30 (0.13 ± 0.041); at muscular/glandular esophagus junction 0.17–0.31 (0.22 ± 0.04), at esophagus-intestinal junction 0.30–0.62 (0.44 ± 0.10). Cephalic extremity with one pair of cuticularized parastomal structures. Four pairs of cephalic papillae. Cephalic plate 38–65 (50 ± 8) µm long, 17–27 (21 ± 2) µm wide. Esophagus total length 1.39–2.73 (1.99 ± 0.36); muscular portion 0.22–0.31 (0.28 ± 0.02) long, 0.03–0.05 (0.04 ± 0.008) wide; glandular portion 1.14–2.41 (1.71 ± 0.34) long, 0.04–0.11 (0.08 ± 0.01) wide; ratio length glandular to muscular 1:0.12–0.22 (0.16 ± 0.025). Nerve ring 0.2–0.30 (0.23 ± 0.03) and vulva 1.23–1.95 (1.53 ± 0.20) from anterior end, respectively. Vagina uterine extending in the glandular region of esophagus, near to junction of muscular and glandular portions. Tail 0.36–0.68 (0.47 ± 0.11) long; width at anus level 0.36–1.14 (0.26 ± 0.07). Lateral and caudal alae present.
Taxonomic summary
Type host: Lithobates brownorum Sanders. Specimens deposited: CNAR 31540–31544.
Site of infection: Body cavity.
Type locality: Calakmul, Campeche, Mexico (18º 48′ 25.9″ N, 89º 44′ 25″ W).
Other localities: Yum Balam, Quintana Roo; Lagunas de Yalahau, Yucatan
Prevalence of infection: 22 of 40 examined (55%).
Type specimens deposited: CNHE 11690, holotype, host CNAR 31542; CNHE 11691 & 11692, paratypes.
GenBank accession number: OR264545-71(COI)
Zoo Bank registration: 231B4DA5-D797-48CB-828F-3C683A0528E9
Etymology: The name of the new species refers to the collection locality, nearby the ancient Mayan city of Calakmul, Campeche, Mexico.
Remarks
Foleyellides calakmulensis n. sp. is included in the genus based on morphological characters referred by Esslinger (1986) and Gibbons (2010), such as the presence of cuticularized parastomal structures, lateral and caudal alae in both sexes, and the lack of distinct cuticularized buccal capsule or annular bands of longitudinally oriented bosses on the cuticle of midbody region.
The new species differs from some other species of Foleyellides (F. americana, F. brachyoptera, F. confusa, F. flexicauda, F. malayensis, F. mayenae, F. ranae, and F. rhinellae) in the number of male caudal papillae, four pairs in the new species and more than four in the other species (García-Prieto et al., 2014; Romero-Mayén & León-Règagnon, 2016; Schmidt & Kuntz, 1969; Wehr & Causey, 1939; Witenerg & Gerichter, 1944). The new species also differs from F. brachyoptera, F. confusa and F. mayenae in the presence of a distinctive cuticularized preanal plaque, which is absent in those species (García-Prieto et al., 2014; Romero-Mayén & León-Règagnon, 2016; Schmidt & Kuntz, 1969). On the other hand, the new species resembles F. duboisi, F. dolichoptera and F. striatus, by having males with four pairs of caudal papillae (Esslinger, 1986; Wehr & Causey, 1939; Witenerg & Gerichter, 1944). However, in F. duboisi and F. dolichoptera the preanal plaque is absent, contrasting with F. calakmulensis. The new species most closely resembles F. striatus in the number of papillae and in the presence of a preanal plaque, but they differ in several features: 1) the size of females, which are smaller in the new species (38–74 in F. striatus vs 19–49 in the new species); 2) the difference in the size of left spicule, which is longer in F. striatus (336–465 vs 265 – 375); 3) the size of papillae (post-anal papillae are the same size in F. striatus (Esslinger, 1986), while different in size in the new species). These combined characteristics distinguish F. calakmulensis n. sp. from F. striatus and from the other described species of the genus.
Genetic distances and phylogenetic analyses
Specimens of two potentially new species of Foleyellides were collected during this study in Yucatan and Quintana Roo, Mexico (table 1); nevertheless, only female specimens were found in spite of intensive collecting efforts in both Mexican states. Without the male characters, those species can not be described in this study, and only COI sequences are presented. Genetic distances of the mitochondrial COI sequences of specimens of F. calakmulensis n. sp. from the same individual host and type locality range from 0 to 0.05%, and 0 to 0.70% between different localities. Genetic distances between F. calakmulensis and F. striatus range from 12.6–13.1%; 12.5–13.2 from F. mayenae, 15.5% from F. rhinellae, 10.5–11.5% from Foleyellides sp. 1 and 14.3–14.5% from Foleyellides sp. 2. Phylogenetic analysis results are presented in figure 3.
Discussion
Eleven species of Foleyellides have been described in the world, the majority of which have been recorded in North America. Ten species are parasites of frogs of the family Ranidae and only F. rhinellae of toads (Rhinella marina) (García-Prieto et al., 2014); all of them inhabit the body cavity of the host, with exception of F. confusa which is subcutaneous (Schmidt & Kuntz, 1969). Taxonomy of the genus Foleyellides is mainly based on morphological characters (body size, number of caudal papillae, presence of cuticularized preanal plaque and size of the spicules); however, many of these characters are variable, and in some cases are difficult to distinguish between species. For example, males of F. calakmulensis n. sp. and F. striatus both have four pairs of papillae, and only with scanning electron microscopy it was possible to clearly corroborate that they are different in size (see Velarde-Aguilar, 2014).
In this sense, molecular tools are important for the differentiation and delimitation of species. We obtained COI sequences of F. calakmulensis n. sp., F. rhinellae, F. striatus, Foleyellides sp. 1 and Foleyellides sp. 2 (table 1), and compared them with sequences of F. mayenae, which were the only available sequences of the genus in GenBank, in order to corroborate the validity of the new species. We also included sequences of other species in the subfamily Waltonellinae: Neofoleyellides boerewors, N. steyni, N. martini (Kuzmin et al., 2021; Netherlands et al., 2020) and three unidentified samples of Ochoterenella (Lefoulon et al., 2015). In the phylogenetic analysis, F. calakmulensis n. sp. appears within a highly supported clade that includes species collected from Lithobates spp. in Jalisco (F. mayenae and F. striatus), Yucatán (Foleyellides sp. 1) and Quintana Roo (Foleyellides sp. 2) (fig. 3). Foleyellides rhinellae, the only species of the genus described parasitizing toads, appears nested within samples of Ochoterenella, a genus that has been typically found in this group of hosts. Further morphological and molecular information would be needed to revise the phylogenetic position and taxonomy of F. rhinellae and to determine if this species should be transferred to Ochoterenella. All other species of Foleyellides included in the analysis are parasites of frogs in the genus Lithobates. It is interesting to note that species that share morphological traits (four pairs of papillae in F. striatus and F. calakmulensis n. sp.) or share geographical distribution and host species (Foleyellides calakmulensis n. sp., Foleyellides sp. 1 and Foleyellides sp. 2, distributed in the Yucatan peninsula) are not sister species to each other in the tree. Further investigation on the phylogenetic relationships among species of Waltonaellinae is needed.
The new species is distributed only in south-eastern Mexico (Campeche, Quintana Roo and Yucatan), although additional geographic sampling is needed in order to determine the geographical distribution of the species in this genus, and also sequences of additional genes are needed to elucidate the evolution of morphological traits and host specificity of Foleyellides species.
References
Anderson, R., & Bain, O. (1976). Keys to genera of the order Spiruruda Part 3. Diplotriaenoidea, Aproctoidea and Filarioidea. In R. Anderson, S. Chabaud, S. Willmott (Eds.), CIH Keys to Nematode parasites Vertebrates. CABI Publishing.
Bain, O. & Prod’hon, J. (1974). Homogenéité des filaires de batracien des genres Waltonella, Ochoterenella et Madochotera; création des Waltonellinae n. subfam. Annales de Parasitologie Humaine Comparée, 45(1), 721–739.
Caballero, E. (1935). Nematodos parásitos de los batracios de México III. Cuarta contribución al conocimiento de la parasitologia de Rana montezumae. Anales del Instituto de Biología Universidad Nacional Autónoma de México, 6(1), 103–117.
Esslinger, J. (1986). Redescription of Foleyellides striatus (Ochoterena and Caballero, 1932) (Nematoda: Filarioidea) from a Mexican frog, Rana montezumae, with reinstatement of the genus Foleyellides Caballero, 1935. Helminthological Society of Washington, 53(1), 218–223.
García-Prieto, L., Ruiz-Torres, N., Osorio-Sarabia, D., & Merlo-Serna, A. (2014). Foleyellides rhinellae sp. nov. (Nematoda, Onchocercidae) a new filaria parasitizing Rhinella marina (Anura, Bufonidae) in Mexico. Acta Parasitologica, 59(1), 478–484. https://doi.org/10.2478/s11686-014-0265-8.
Gibbons, L. (2010). Keys to the Nematode Parasites of vertebrates, CABI Publising Wallingford, UK.
Katoh, K., & Standley, D. (2013). MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Molecular Biology and Evolution, 30(1), 772–780. https://doi.org/10.1093/molbev/mst010.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35(1), 1547–1549. https://doi.org/10.1093/molbev/msy096.
Kuzmin, Y., Netherlands, E., du Preez, L., & Svitin, R. (2021). Two new species of Neofoleyellides (Nematoda: Onchocercidae) parasitising anuran amphibians in South Africa. International Journal for Parasitology: Parasites of Wildlife, 14(1), 298–307. org/https://doi.org/10.1016/J.IJPPAW.2021.02.018.
Lefoulon, E., Bain, O., Bourret, J., Junker, K., Guerrero, R., Cañizales, I., Kuzmin, Y., Satoto, T., Cardenas-Callirgos, J., De Souza Lima, S., Raccurt, C., Mutafchiev, Y., Gavotte, L., & Martin, C. (2015). Shaking the tree: Multi-locus sequence typing usurps current onchocercid (Filarial Nematode) phylogeny. PLoS Neglected Tropical Diseases, 9(11), e0004233. https://doi.org/10.1371/JOURNAL.PNTD.0004233.
Lima-Monteiro, K., Jaeger, L., Coronato, B., Aparecida, D., Chaves, R., Araújo, P., Alves, B., Pereira, J., Neves, F., & Carvalho-Costa, F. (2018). Mitochondrial DNA reveals species composition and phylogenetic relationships of hookworms in northeastern Brazil. Infection Genetics and Evolution, 68(1), 105–112.https://doi.org/10.1016/j.meegid.2018.11.018.
López-Neyra, R. C. (1956). Revisión de la Superfamilia Filarioidea (Weinland, 1858). Revista Ibérica de Parasitologia, 16(1), 2–223.
Nadler, S. (2002). Species delimitation and nematode biodiversity: Phylogenies rule. Nematology, 4(1), 615–625. org/https://doi.org/10.1163/15685410260438908.
Netherlands, E., Svitin, R., Cook, C., Smit, N., Brendonck, L., Vanhove, M., & Du Preez, L. (2020). Neofoleyellides boerewors n. gen. n. sp. (Nematoda: Onchocercidae) parasitising common toads and mosquito vectors: morphology, life history, experimental transmission and host-vector interaction in situ. International Journal for Parasitology, 50(1), 177–194. https://doi.org/10.1016/j.ijpara.2019.11.009.
Ochoterena, I., & Caballero, E. (1932). Una nueva filaria parásita de las ranas. Anales del Instituto de Biología Universidad Nacional Autónoma de México, 3(1), 29–32.
Posada, D. (2008). jModelTest: Phylogenetic model averaging. Molecular Biology and Evolution, 25(1), 1253–1256. https://doi.org/10.1093/molbev/msn083.
Powers, T., Harris, T., Higgins, R., Mullin, P., & Powers, K. (2018). Discovery and identification of Meloidogyne species using COI DNA barcoding. Journal of Nematology, 50(1), 399–412. https://doi.org/10.21307/jofnem-2018-029.
Powers, T., Harris, T., Higgins, R., Mullin, P., Sutton, L., & Powers, K. (2011). MOTUs, morphology, and biodiversity estimation: A case study using Nematodes of the suborder Criconematina and a conserved 18S DNA barcode. Journal of Nematology, 43(1), 35–48.
Prosser, S., Velarde-Aguilar, M., León-Règagnon, V., & Hebert, P. (2013). Advancing nematode barcoding: A primer cocktail for the cytochrome c oxidase subunit I gene from vertebrate parasitic nematodes. Molecular Ecology Resources, 13(1), 1108–1115. https://doi.org/10.1111/1755-0998.12082.
Romero-Mayén, A., & León-Règagnon, V. (2016). A new species of Foleyellides (Nematoda: Onchocercidae) parasite of Lithobates spp. (Amphibia: Ranidae) from Mexico with a key for the species of genus. Zootaxa, 4170(1), 581–586. https://doi.org/10.11646/zootaxa.4170.3.10.
Ronquist, F., Teslenko, M., Van Der Mark, P., Ayres, D., Darling, A., Höhna, S., Larget, L., Liu, L., Suchard, M., & Huelsenbeck, J. (2012). MrBayes 3.2: Efficient bayesian phylogenetic inference and model choice across a large model space, Systematic Biology, 61(1), 539–542. https://doi.org/10.1093/sysbio/sys029.
Schmidt, G., & Kuntz, E., (1969). Nematode parasites of Oceanica: VI. Foleyella confusa sp.nov., Icosiella hoogstraali sp.nov. (Filarioidea), and other species from Philippine amphibians, Parasitology, 59(1), 885–889. https://doi.org/10.1017/S0031182000070396.
Siddall, M., Kvist, S., Phillips, A., & Oceguera-Figueroa, A. (2012). DNA Barcoding of Parasitic Nematodes: Is it Kosher?. Journal of Parasitology, 98(1), 692–694.
Velarde-Aguilar, G. (2014). Estudio filogenético y helmintológico de Lithobates megapoda en el estado de Jalisco. Maestría en Ciencias Biologicas, Thesis. Universidad Nacional Autónoma de México. http://132.248.9.195/ptd2014/agosto/0717928/Index.html
Wehr, E., & Causey, O. (1939). Two new nematodes (Filarioidea: Dipetalonematidae) from Rana sphenocephala. American Journal of Epidemiology, 30(1), 65–68. https://doi.org/10.1093/oxfordjournals.aje.a118602.
Witenerg, G., & Gerichter, C. (1944). The morphology and life history of Foleyella duboisi, with remarks on allied filariids of amphibia. Journal of the Parasitology, 30(1), 245–256.
Yamaguti, S. (1961). The nematodes of vertebrates. Interscience. New York.
Acknowledgments
We thank Posgrado en Ciencias Biológicas UNAM and Consejo Nacional de Ciencia y Tecnología (CONACYT) for the support and scholarships to YV-U, and MGVA. Special thanks to Julio Cesar Canales, Amparo Rodríguez, Sergio Guillén, Estela Muñoz, Mariel Montes, Enrique Silva and Kary López for their help in the collection of specimens; Nallely Ruiz-Torres for donating F. rhinellae tissue samples; Ofelia Delgado, for her help with the molecular analyses; Laura Márquez and Berenit Mendoza at Laboratorio Nacional de la Biodiversidad (LANABIO), for their help with sequencing of samples and the SEM images respectively; Luis García-Prieto for reviewing the manuscript.
Funding
This study was funded by CONACYT project 220408 to VLR and Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT) 202029 to AOF.
Author information
Authors and Affiliations
Contributions
YV-U, AO-F and VL-R conceived and designed the study; YV-U and MGV-A collected the specimens and did the observations; YV-U did the analysis; YV-U wrote the main draft and prepared images; all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical standards
All applicable institutional, national and international guidelines for animal care and use were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Velázquez-Urrieta, Y., Velarde-Aguilar, M.G., Oceguera-Figueroa, A. et al. New species of Foleyellides (Nematoda: Onchocercidae: Waltonellinae), parasite of Lithobates brownorum (Amphibia: Ranidae) from South-eastern Mexico and genetic barcodes of the Mexican species of the genus. Syst Parasitol 100, 591–599 (2023). https://doi.org/10.1007/s11230-023-10108-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-023-10108-1