Abstract
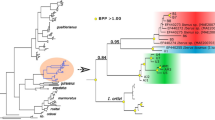
In July 2012 new Allocreadium species was isolated from Carassius gibelio caught in the Arsenyevka River, Primorsky region, Russia. Analyses on the morphometrics and internal organs′ topology revealed that these worms are morphologically closest with A. isoporum but both species are independent according to high genetic distances based on the 28S gene fragment (5.434±0.0073%). Unlike A. isoporum found earlier in Europe, the new species named A. pseudoisoporum sp. nov. has a shorter body length and the vitellarium not reaching the posterior end of the body at some distance and its anterior border is on the level of the ventral sucker. Allocreadium pseudoisoporum sp. nov. differs from seven species previously found in the Russian Far East with the following features: smaller size of the body, suckers′ ratio, range values, and topology of internal structures. Newly localities in the Pavlovka River and the Artyomovka River were discovered for A. khankaiensis. Morphological variability of the worms from the Pavlovka River was observed in comparison with A. khankaiensis from the Komissarovka River. Using scanning electron microscope, we examined external surfaces of three species (A. pseudoisoporum sp. nov., A. khankaiensis, A. hemibarbi) and observed structures reminiscent sensory receptors. This study was aimed to describe species diversity of allocreadiids inhabiting the south of Primorsky region, and to clarify phylogenetic relationships between the species from the genus Allocreadium Looss, 1900 using molecular genetic methods. The phylogenetic Bayesian tree based on the 28S gene showed a clear separation of ten Allocreadium species and confirmed the validity of A. pseudoisoporum sp. nov. Allocreadium pseudoisoporum sp. nov. is most similar to A. gotoi (genetic distances – 3.578±0.0051% in 28S, and 18.777±0.0149% in cox1), and represented the earliest divergent lineage in Allocreadium clade on the phylogenetic tree based on the 28S gene, thereby indicating its proximity to the ancestral node. Also, dichotomous keys for 25 Palearctic species of Allocreadium were prepared based on the morphology of the adult worms.





Similar content being viewed by others
Data availability
All data generated or analysed during this study are included in this published article and its supplementary information files.
References
Abdou, N. E., & Ashour, A. A. (2000). Scanning electron microscopy of the tegumental surface of digenetic trematode Stephanostomum egypticum from the Red Sea fishes. Journal of the Egyptian Society of Parasitology, 30 (1), 341–348.
Achmerov, A. H. (1960). New trematode species from the River Amur. Helminthologia, 2 (3-4), 286–394. [In Russian]
Achmerov, A. H. (1961). To the knowledge of the trematode fauna of fishes in the Amur River basin. Proceedings of GELAN, 11, 22-31. [In Russian]
Achmerov, A. H. (1963). Allocreadium maculati nov. sp. new species of trematode from Amur fish. Trudy Helmintologicheskoy laboratorii AS USSR, 13, 250-252. [In Russian]
Atopkin, D. M., & Shedko, M. B. (2014) Genetic characterization of far eastern species of the genus Crepidostomum (Trematoda: Allocreadiidae) by means of 28S ribosomal DNA sequences. Adv. Biosci. Biotechnol., 5(3), 209-215. https://doi.org/10.4236/abb.2014.53027
Atopkin, D. M., Sokolov, S. G., Shedko, M. B., Vainutis, K. S., & Orlovskaya, O.M. (2018). Diversity of the genus Bunodera Railliet, 1896 (Trematoda: Allocreadiidae) in the northern part of Eastern Europe and North-eastern Asia, estimated from 28S rDNA sequences, with a description of Bunodera vytautasi sp. nov. Parasitology Research, 117(6), 1765-1772. https://doi.org/10.1007/s00436-018-5858-y
Aydogdu, A., Pérez-Ponce de León, G., Emre, Y., Emre, N., & Yabacı, A. (2018). Prevalence and intensity of Allocreadium isoporum (Digenea: Allocreadiidae) in three endemic species of cyprinids (Capoeta spp.) in Turkey, in relation to season, host size and sex. Journal of Applied Ichthyology, 34(5-6). https://doi.org/10.1111/jai.13515
Belle, C. C., Stoeckle, B. C., Cerwenka, A. F., Kuehn, R., Mueller, M., Pander, J., & Geist, J. (2017). Genetic species identification in weatherfish and first molecular confirmation of Oriental Weatherfish Misgurnus anguillicaudatus (Cantor, 1842) in Central Europe. Knowl. Manag. Aquat. Ecosyst., 418, 31.
Berg, L. S. (1949). Freshwater fishes of the U.S.S.R. and adjacent countries. Acad. Sci. Moscow USSR, Vols. 1, 2, 3 (Edn 4).
Bogatov, V. V., & Vainutis, K. S., (2022). About the origin of the family Allocreadiidae (Trematoda: Plagiorchiida). Doklady Biological Sciences, 502(1), 104–108. https://doi.org/10.31857/S268673892201005X
Bray, R. A., Foster, G. N., Waeschenbach, A., & Littlewood, D. T. J. (2012). The discovery of progenetic Allocreadium neotenicum Peters, 1957 (Digenea: Allocreadiidae) in water beetles (Coleoptera: Dytiscidae) in Great Britain. Zootaxa, Vol. 3577, № 1. P. 58–70. https://doi.org/10.11646/zootaxa.3577.1.3
Brooks, D. R., & McLennan, D. A. (1993). A brief history of parasite evolutionary biology. In: V. A. Funk, & P. F. Cannell (Eds.), Parascript: parasites and the language of evolution (1st ed., pp. 5 - 19). Smithsonian Institution Press, Washington and London.
Caira, J. N. (1985). A revision of the North American papillose Allocreadiidae (Digenea) with independent cladistic analyses of larval and adult forms. Bulletin of the University of Nebraska State Museum. PhD Thesis, 11(3), 1–58.
Chandra, S., Yadav, N., & Saxena, A. (2019). Two new records of digenetic trematode genus Allocreadium Looss, 1900 from fresh water fishes of Lucknow, India. Current Life Sciences, 5(2), 28-34. https://doi.org/10.5281/zenodo.3262331
Cheng P., Yu D., Tang Q., Yang J., Chen Y., & Liu H. (2022). Macro-evolutionary patterns of East Asian opsariichthyin-xenocyprinin-cultrin fishes related to the formation of river and river-lake environments under monsoon climate. Water Biology and Security, 1(2), 100036. https://doi.org/10.1016/j.watbs.2022.100036
Chereshnev, I. A. (1998). Biogeography of freshwater fish in the Russian Far East. Vladivostok, Dalnauka. (In Russian)
Choudhury, A., León-Règagnon, V. (2005). Molecular phylogenetics and biogeography of Bunodera spp. (Trematoda: Allocreadiidae), parasites of percid and gasterosteid fishes. Canadian Journal of Zoology, 83(12), 1540–1546. https://doi.org/10.1139/z05-153
Curran, S. S., Tkach, V. V., & Overstreet, R. B. (2006). A review of Polylekithum Arnold 1934 and its familial affinities using morphological and molecular data, with description of Polylekithum catahoulensis sp. nov. Acta Parasitologica, 51(4), 238–248. https://doi.org/10.2478/s11686-006-0037-1
Curran S. S., Tkach V.V., Overstreet R.M. Phylogenetic affinities of Auriculostoma (Digenea: Allocreadiidae), with descriptions of two new species from Peru // Journal of Parasitology. 2011. Vol. 97, № 4. P. 661–670. https://doi.org/10.1645/GE-2641.1
Darriba, D., Taboada, G. L., Doallo, R., & Posada, D. (2012). jModelTest 2: more models, new heuristics and parallel computing. Nat Methods, 9, 772. https://doi.org/10.1038/nmeth.2109
Dos Santos, Q. M., Gilbert, B. M., Avenant-Oldewage, A., & Dumbo, J. C. (2021). Morphological and molecular description of Allocreadium apokryfi sp. n. (Digenea: Allocreadiidae) from native Labeobarbus aeneus (Cyprinidae) in South Africa, including notes on its biology, evolutionary history and an updated key of African Allocreadium. Folia Parasitologica, 68: 013. https://doi.org/10.14411/fp.2021.013
Ermolenko, A. V. (1990). Causative agents of invasive diseases of freshwater fishes from Primorsky Krai. Vladivostok: FEB AS USSR. [In Russian]
Ermolenko, A. V. (1992). Parasites of fishes from freshwater ponds of the continental part of Japan sea basin. Vladivostok: FEB RAS. [In Russian]
Faltýnková, A., Pantoja, C., Skírnisson, K., & Kudlai, O. (2020). Unexpected diversity in northern Europe: trematodes from salmonid fishes in Iceland with two new species of Crepidostomum Braun, 1900. Parasitology Research, 119, 2439–2462. https://doi.org/10.1007/s00436-020-06724-1
Franceschini, L., Aguiar, A., Zago, A. C., de Oliveira Fadel Yamada, P., Bertholdi Ebert, M., & da Silva, R. J. (2021). Three new species of Creptotrema (Trematoda, Allocreadiidae) with an amended diagnosis of the genus and reassignment of Auriculostoma (Allocreadiidae), based on morphological and molecular evidence. Parasite, 28, 69. https://doi.org/10.1051/parasite/2021065
Gao, D., Wang, G. T., Xi, B. W., Yao, W. J., & Nie, P. (2008). A New Species of Allocreadium (Trematoda: Allocreadiidae) from Freshwater Fishes in the Danjiangkou Reservoir in China. Journal of Parasitology, 94(1), 176-180. https://doi.org/10.1645/ge-1247.1
Hasegawa, T. & Ozaki, Y. (1926). On a new trematode parasitic in Misgurnus anguillicaudatus. Dobutsugaku Zasshi, 38, 225–228. [In Japanese]
Hernández-Mena, D. I., Lynggaard, C., Mendoza-Garfias, B., & Pérez-Ponce de León, G. (2016). A new species of Auriculostoma (Trematoda: Allocreadiidae) from the intestine of Brycon guatemalensis (Characiformes: Bryconidae) from the Usumacinta River Basin, Mexico, based on morphology and 28S rDNA sequences, with a key to species of the genus. Zootaxa, 4196(2), 261–277. https://doi.org/10.11646/zootaxa.4196.2.5
Hernández-Mena, D. I., Pinacho-Pinacho, C. D., García-Varela, M., Mendoza-Garfias, B., & de León, G.P. (2019). Description of two new species of allocreadiid trematodes (Digenea: Allocreadiidae) in middle American freshwater fishes using an integrative taxonomy approach. Parasitology Research, 118(2), 421–432. https://doi.org/10.1007/s00436-018-6160-8
Huelsenbeck, J. P., Ronquist, F., Nielsen, R., & Bollback, J.P. (2001). Bayesian inference of phylogeny and its impact on evolutionary biology. Science, 294, 2310–2314. https://doi.org/10.1126/science.1065889
Kaufman, D., Walter, R., Brigham-Grette, J., & Hopkins, D. (1991). Middle Pleistocene age of the Nome River glaciation, northwestern Alaska. Quaternary Research, 36(3), 277-293. https://doi.org/10.1016/0033-5894(91)90003-N
Koshelev, V. N., Kolpakov, N. V. (2020). Sakhalin sturgeon Acipenser mikadoi (Acipenseridae): results of study and proposed measures for conservation of the species. Izvestiya TINRO, 200(4):791-808. https://doi.org/10.26428/1606-9919-2020-200-791-808 [In Russian]
Koval, V. P. (1957). Trematodes of the genus Allocreadium Looss, 1900 in fishes of freshwater ponds of UkSSR. Naukovi zapiski Kiivs′kogo Universitetu, v. XVI, XX. Biologichniy zbirnik, 14, 201-212. [In Ukrainian]
Koval, V. P. (1966). Family Allocreadiidae Stossich, 1903. In K. I. Skryabin (Ed.), Trematodes of animals and men (vol. 22, pp. 185–305). Nauka. (In Russian)
Kozicka, I. (1956). Parazyty ryb jeziora Goldapiwo. Wiadom. parazytol., 2(5), 207. [In Polish]
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Molecular biology and evolution, 35(6), 1547–1549. https://doi.org/10.1093/molbev/msy096
Lasee, B. A., Font, W. F., & Sutherland, D. R. (1988). Culaeatrema inconstans gen.n., sp.n. (Digenea: Allocreadiidae) from the brook stickleback (Culaea inconstans) in Wisconsin and observations on parthenogenetic populations. Canadian Journal of Zoology, 66, 1328–1333. https://doi.org/10.1139/z88-195
Layman, E. M. (1933). Parasitic worms of fishes of Lake Baikal. Tr. Baikalsk. limnol. stantsii, 4, 5-98. [In Russian]
Lindberg, G. U. (1955). Quaternary Period in the Light of Biogeographic Data. Publishing House of the USSR Academy of Sciences, Leningrad, Monograph. [In Russian]
Linstow, O. F. B. (1896). Helminthologische Mitteilungen. Arch. Microskop. Anat., 48(3), 373-397.
Lobanov, A. L., Kirejtshuk, A. G., Stepanjants, S. D., & Smirnov, I. S. (2013). Diagnostic keys: from the textual dichotomous to computer ones. Proceedings of the Zoological Institute RAS, Appendix No. 2, 249–268.
Lockyer, A. E., Olson, P. D., & Littlewood, D. T. J. (2003). Utility of complete large and small subunit rRNA genes in resolving the phylogeny of the Neodermata (Platyhelminthes): implications and a review of the cercomer theory. Biol. J. Linn. Soc. Lond., 78, 155–171. https://doi.org/10.1046/j.1095-8312.2003.00141.x
Looss, A. (1894). Die Distomen unserer Fische und Frösche Neue Untersuchungen über Bau und Entwickelung des Distomenkörpers. Bibl. Zool., V. 16, 296 p.
Lozhkin, A. V., Anderson, P., Eisner, W. R., & Solomatkina, T. B. (2011). Late glacial and holocene landscapes of central Beringia. Quaternary Research, 76(3), 383–392. https://doi.org/10.1016/j.yqres.2011.08.003
Lühe, M. (1909). Parasitische Plattwurmer. I. Trematodes. In A. Brauer (Ed.), Die Süsswasserfauna Deutschlands. Eine Exkursionfauna (vol. 2, is. 17, p. 217). Jena, Gustav Fischer.
Martínez-Aquino, A., Ceccarelli, F. S., & de León, G. P.-P. (2013). Molecular phylogeny of the genus Margotrema (Digenea: Allocreadiidae), parasitic flatworms of goodeid freshwater fishes across central Mexico: species boundaries, host-specificity, and geographical congruence. Zoological Journal of the Linnean Society, 168(1), 1–16. https://doi.org/10.1111/zoj.12027
MacIntosh, A. J. J., & Frias, L. (2017). Coevolution of hosts and parasites. In A. Fuentes (Ed.), The international encyclopedia of primatology (vol. 1, pp. 188–196). Chichester, Wiley/Blackwell.
Manter, H. W. (1963). The Zoogeographical Affinities of Trematodes of South American Freshwater Fishes. Systematic Biology, 12(2), 45–70. https://doi.org/10.2307/sysbio/12.2.45
Martínez-Aquino, A., Ceccarelli, F. S., Eguiarte, L. E., Vázquez-Domínguez, E., & de León, G. P.-P. (2014). Do the Historical Biogeography and Evolutionary History of the Digenean Margotrema spp. across Central Mexico Mirror Those of Their Freshwater Fish Hosts (Goodeinae)? PLoS ONE, 9(7), e101700. https://doi.org/10.1371/journal.pone.0101700
Milton, J., Paray, B. A., & Rather, I. (2018). A review on the biology and physiology of loach Misgurnus anguillicaudatus in China. Indian Journal of Geo-Marine Sciences, 47(4), 759–765.
Miura, F., Uematsu, C., Sakaki, Y., & Ito, T. (2005). A novel strategy to design highly specific PCR primers based on the stability and uniqueness of 3′-end subsequences. Bioinformatics, 21, 4363–4370.
Montes, M. M., Barneche, J., Croci, Y., Balcazar, D., Almirón, A., Martorelli, S., & Pérez-Ponce de León, G. (2021). Description of a new species of Auriculostoma (Digenea: Allocreadiidae) from Characidium heirmostigmata (Characiformes: Crenuchidae) from Argentina, using morphological and molecular data. Journal of Helminthology, 95, e19, 1–8. https://doi.org/10.1017/S0022149X21000109
Montes, M. M., Barneche, J., Croci, Y., Rodriguez Gil, S., Curran, S. S., Ferrari, W., Casciotta, J. R. & Martorelli, S. R. (2020). Prosthenhystera gattii n. sp. (Digenea: Callodistomidae), a gallbladder parasite of Bryconamericus ikaa from the lower Iguazú River, described based on combined molecular and morphological evidence. Journal of helminthology, 94, e151. https://doi.org/10.1017/S0022149X20000322
Moravec, F. (1984). Systematic status of Allocreadium carparum Odening, 1959 (Digenea) and three other related species from fishes of Czechoslovakia. Folia Parasitologica, 31, 215–224.
Moravec, F. (1992). Observations on the bionomy of Allocreadium isoporum (Looss, 1894) (Trematoda: Allocreadiidae). Folia Parasitologica, 39(2), 133–144.
Moravec, F. (2002). External morphological differences between Crepidostomum farionis and Crepidostomum metoecus (Trematoda: Allocreadiidae), parasites of salmonids, as revealed by SEM. Folia Parasitologica, 49(3), 211–217. https://doi.org/10.14411/fp.2002.037
Morgan, J. A. T., & Blair, D. (1998). Relative merits of nuclear ribosomal internal transcribed spacers and mitochondrial CO1 and ND1 genes for distinguishing among Echinostoma species (Trematoda). Parasitology, 116, 289–297.
Near, T. J., Eytan, R. I., Dornburg, A., Kuhn, K. L., Moore, J. A., Davis, M. P., Wainwright, P. C., Friedman, M., & Smith, W. L. (2012). Resolution of ray-finned fish phylogeny and timing of diversification. Proceedings of the National Academy of Sciences, 109(34), 13698–13703. https://doi.org/10.1073/pnas.1206625109
Okino, T., Ushirogawa, H., Matoba, K., & Ohyama, F. (2004). Survey of parasitic helminths in loaches imported from China. Japanese Journal of Zoo and Wildlife Medicine, 9, 71–78. [In Japanese with English abstract]
Olson, P. D., Cribb, T. H., Tkach, V. V., Bray, R. A., & Littlewood, D. T. J. (2003). Phylogeny and classification of the Digenea (Platyhelminthes: Trematoda). International Journal for Parasitology, 33(7), 733–755.
Ozaki, Y. (1926). On some new species of trematodes of freshwater fishes from Japan (Preliminary report). Dobutsugaku Zasshi, 38, 124–130. [In Japanese]
Pérez-Ponce De León, G., Martínez-Aquino, A., & Mendoza-Garfias, B. (2013). A new species of Margotrema (Digenea, Allocreadiidae) from the leopard splitfin Xenotaenia resolanae (Cyprinodontiformes, Goodeidae) from west-central Mexico. Zootaxa, 3670(1), 94–96. https://doi.org/10.11646/zootaxa.3670.1.10
Pérez-Ponce de León, G., Pinacho-Pinacho, C. D., Mendoza-Garfias, B., Choudhury, A., & Garcia-Varela, M. (2016). Phylogenetic analysis using the 28S rRNA gene reveals that the genus Paracreptotrema Choudhury, Pérez-Ponce de León, Brooks and Daverdin, 2006 (Digenea: Allocreadiidae) is not monophyletic; description of two new genera and one new species. Journal of Parasitology, 102(1), 131–142. https://doi.org/10.1645/15-815
Pérez-Ponce de León, G., Sereno-Uribe, A., García-Varela, M., Mendoza-Garfias, B., Hernández-Mena, D., Pinacho-Pinacho, C., & Choudhury, A. (2020). Disentangling the evolutionary and biogeographical history of the freshwater fish trematode genus Creptotrema (Digenea: Allocreadiidae) using an integrative taxonomy approach: The case of Creptotrema agonostomi in Middle American mountain mullets. Journal of Helminthology, 94, E171. https://doi.org/10.1017/S0022149X2000053X
Petkevičiūtė, R., Stunzenas, V., Staneviciute, G., & Sokolov, S. G. (2010). Comparison of the developmental stages of some European allocreadiid trematode species and a clarification of their life-cycles based on ITS2 and 28S sequences. Systematic Parasitology, 76(3), 169–178.
Petkevičiūtė, R., Stunžėnas, V., & Stanevičiūtė, G. (2012). Clarification of the systematic position of Cercariaeum crassum Wesenberg-Lund, 1934 (Digenea), based on karyological analysis and DNA sequences. Journal of Helminthology, 86(3), 293–301. https://doi.org/10.1017/S0022149X11000393
Petkevičiūtė, R., Stunžėnas, V., Zhokhov, A. E., Poddubnaya, L. G., & Stanevičiūtė, G. (2018). Diversity and phylogenetic relationships of European species of Crepidostomum Braun, 1900 (Trematoda: Allocreadiidae) based on rDNA, with special reference to Crepidostomum oschmarini Zhokhov & Pugacheva, 1998. Parasites & Vectors, 11, 530.
Razo-Mendivil, U., Mendoza-Garfias, B., Pérez-Ponce de León, G., & Rubio-Godoy, M. (2014). A new species of Auriculostoma (Digenea: Allocreadiidae) in the Mexican tetra Astyanax mexicanus (Actinopterygii, Characidae) from Central Veracruz, Mexico, described using morphological and molecular data. Journal of Parasitology, 100, 331–337. https://doi.org/10.1645/13-352.1
Rohde, K., & Watson, N. (1989). Sense receptors in Lobatostoma manteri (Trematoda, Aspidogastrea). International Journal for Parasitology, 19(8), 847–858. https://doi.org/10.1016/0020-7519(89)90110-0
Roitman, V. A. (1963). New species of trematodes of fishes of the Amur basin. Trudy Gel′mintologicheskoi Laboratorii, 13, 303–312. [In Russian]
Saitoh, K., Sado, T., Doosey, M. H., Bart, Jr, H. L., Inoue, J. G., Nishida, M., Mayden, R. L., & Miya, M. (2011). Evidence from mitochondrial genomics supports the lower Mesozoic of South Asia as the time and place of basal divergence of cypriniform fishes (Actinopterygii: Ostariophysi). Zoological Journal of the Linnean Society, 161(3), 633–662. https://doi.org/10.1111/j.1096-3642.2010.00651.x
Shimazu, T. (1988). Trematodes of the genus Allocreadium (Allocreadiidae) from a freshwater fish in Nagano, Central Japan. Bull. Natl. Sci. Mus. Tokyo Ser. A. Zool., (14) 1–21.
Shimazu, T. (2016). Digeneans Parasitic in Freshwater Fishes (Osteichthyes) of Japan VII. Allocreadiidae: Allocreadium, Bulletin of the National Museum of Nature and Science, Series A (Zoology), 42(2), 55–79.
Shimazu, T. (2017). Digeneans parasitic in freshwater fishes (Osteichthyes) of Japan XII. A list of the papers of the series, a key to the families in Japan, a parasite-host list, a host-parasite list, Addenda, and Errata. Bull. Natl. Mus. Nat. Sci. Ser. A, 43, 129-143.
Shtein, G. A. (1957). Materials on the parasite fauna of aquatic arthropods from some Karelian lakes. II. Digenetic trematodes (Trematoda), metacercariae. Uch. Zap. Petrozavodsk. Gos. Univ., 8, 120-139. [In Russian]
Slusarski, W. (1958). Formy ostateczne Digenea z ryb lososiowatych (Salmonidae) dorzecza Wisly I Poludniowegu Baltyky. Acta Parasitologica Polonica, 6(22), 247-528. [In Polish]
Strelkov, Yu. A. (1971). Digenean flukes of fishes from Amur River basin. In Parasitological collection of Zoological Institute AS USSR (Vol. 25, pp. 120-139). Nauka, Leningrad. (In Russian)
Tkach, V. V., & Curran, S. S. (2015). Prosthenystera oonastica n. sp. (Digenea: Callodistomidae) from ictalurid catfishes in southeastern United States and molecular evidence differentiating species in the genus across Americas. Systematic Parasitology, 90(1), 39–51. https://doi.org/10.1007/s11230-014-9531-2
Tkach, V. V., Curran, S. S., Bell, J. A., & Overstreet, R. M. (2013). A new species of Crepidostomum (Digenea: Allocreadiidae) from Hiodon tergisus in Mississippi and molecular comparison with three congeners. Journal of Parasitology, 99, 1114–1121. https://doi.org/10.1645/13-279.1
Tkach, V. V., Littlewood, D. T. J., Olson, P. D., Kinsella, M., & Swiderski, Z. (2003). Molecular phylogenetic analysis of the Microphalloidea Ward, 1901 (Trematoda: Digenea). Systematic Parasitology, 56(1), 1-15. https://doi.org/10.1023/A:1025546001611
Truett, G. E. (2006). Preparation of genomic DNA from animal tissues. In J. Kieleczawa (Ed.), The DNA book: protocols and procedures for the modern molecular biology laboratory (pp. 33–46). Jones and Bartlett Publisher, Sudbury.
Urabe, M., Hashim, N. E. N., Uni, S., Iwaki, T., Halim, M. R. A., Marzuki, M. E., Udin, A. S. M., Zainuri, N. A., Omar, H., Agatsuma, T., Uga, S., Takaoka, H., Azirun, M. S., Ramli, R. (2020). Description and molecular characteristics of Morishitium polonicum malayense Urabe, Nor Hashim & Uni, n. subsp. (Trematoda: Cyclocoelidae) from the Asian glossy starling, Aplonis panayensis strigata (Passeriformes: Sturnidae) in Peninsular Malaysia. Parasitology International, 76, 102074. https://doi.org/10.1016/j.parint.2020.102074
Vainutis K. S. (2020). Allocreadium khankaiensis sp. nov. and Allocreadium hemibarbi Roitman, 1963 (Trematoda: Allocreadiidae) from the Russian Far East: Morphological, molecular, and phylogenetic studies. Parasitology International, 76: 102102. https://doi.org/10.1016/j.parint.2020.102102
Vainutis, K. S., Voronova, A. N., & Urabe, M. (2021). Systematics of Crepidostomum species from the Russian Far East and northern Japan, with description of a new species and validation of the genus Stephanophiala // Parasitology International, 84, 102412. https://doi.org/10.1016/j.parint.2021.102412
Vdovin, A. N., Zuenko, Yu. I., Solomatov, S. F., Bolshakov, S. G. (2015). Dynamics of Far-Eastern dace Tribolodon brandtii in the Amur bay (Peter the Great bay, Japan sea). Problems of fisheries, 16(1), 107-117. [In Russian]
Wishniewski, L. W. (1958). The development of cycle of Bunodera luciopercae (O. F. Muller). Acta Parasitol. Polon. 6(11), 290-305.
Žďárská, Z., & Nebesářová, J. (2003). Transmission electron microscopy of intra-tegumental sensory receptors in the forebody of Crepidostomum metoecus (Digenea: Allocreadiidae). Folia Parasitologica, 50, 215–219.
Žďárská, Z., & Nebesářová, J. (2004). Transmission electron microscopy of presumed sensory receptors in the forebody papillae of Crepidostomum metoecus (Digenea: Allocreadiidae). Folia Parasitologica, 51(1), 27-32. https://doi.org/10.14411/FP.2004.004
Zimmerman, C. E., Rand, P. S., Fukushima, M., & Zolotukhin, S. F. (2012). Migration of Sakhalin taimen (Parahucho perryi): evidence of freshwater resident life history types. Environmental Biology of Fishes, 93, 223–232. https://doi.org/10.1007/s10641-011-9908-x
Acknowledgements
The authors are grateful to the head of the Parasitology Department Dr. Vladimir V. Besprozvannykh (Federal Scientific Center of the East Asia Terrestrial Biodiversity FEB RAS) for providing the material collected in the Arsenyevka River in 2012.
Funding
The research was carried out within the state assignment of Ministry of Science and Higher Education of the Russian Federation (theme No. 121031000154-4).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Vainutis, K.S., Voronova, A.N., Urabe, M. et al. Integrative approach for discovering of the new species within the genus Allocreadium Looss, 1900 (Trematoda: Allocreadiidae) and framing of biogeographical hypotheses for the genus. Syst Parasitol 100, 189–213 (2023). https://doi.org/10.1007/s11230-022-10081-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-022-10081-1