Abstract
The Iberian Peninsula constitutes a diversity hotspot with a high number of endemisms, where the land snail genus Iberus is likely the best example. Despite this, its species diversity is still debated as it holds several cryptic species. In the present paper, we use molecular evidence (mitochondrial DNA cytochrome oxidase subunit I) to clarify the position of I. ortizi and three new cryptic species that are described herein: I. giennensis, I. axarciensis and I. antikarianus spp. nov. For this, we sampled 281 sampling points to delimitate a comprehensive geographic mapping of these species. Moreover, we carried out a comprehensive morphometric analysis based on 3205 shells. Our findings show that, morphologically, the three described species overlap in the form of their shells, their morphologies being very similar to other close species with nearby distributions (I. ortizi, I. angustatus and I. marmoratus loxanus). Still, all these species are well-defined by genetic distances, but display allopatric distributions, suggesting that they evolved by allopatric speciation as a consequence of biogeographic isolation. Hence, our findings show insights into the evolution of land snails in southeastern Spain, with implications for their conservation, given that our exhaustive sampling shows that the three species described here have very limited distribution ranges, especially I. antikarianus sp. nov. Our study, moreover, implies an integrated approach to the study of the evolution of land snails, including the sampling of the complete geographic area occupied by the genus, genetic analysis to delimit the actual species range, as well as morphometric analyses to understand the phenotypic differentiation and adaptations of the three new species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Iberian Peninsula constitutes a diversity hotspot with many endemic species, largely boosted by its geographical location and turbulent geological history. The Iberian Peninsula serves as a bridge between African and Eurasian faunas (Husemann et al., 2014) and was a glacial refuge favouring ulterior speciation processes (Abellán & Svenning, 2014). Moreover, the mountainous topography of the Iberian Peninsula, acting like biogeographic islands and barriers, has also contributed to the emergence and development of endemic species (López-Villalta, 2011).
Within the endemic species in the Iberian Peninsula, the land snails stand out for their high number of endemics (Cadevall & Orozco, 2016), with the genus Iberus Monfort, 1810 being the most representative Iberian endemic land snail. However, the species diversity of the genus Iberus is still debated. The species type for the genus is Helix gualtierana Linnaeus, 1758, currently designed as Iberus gualtieranus (Linnaeus, 1758). The taxonomy of the genus was revised by García San Nicolás (1957) based on morphological characters. Nevertheless, subsequent studies based on molecular techniques (sequencing of the cytochrome oxidase subunit I [COI] and RNA ribosomal 16S [16S rRNA]) showed that the morphology is of limited use to delimit species (Elejalde et al., 2005, 2008a, b). The genus Iberus includes cryptic species such as I. ortizi García San Nicolás, 1957 or I. marmoratus (A. Férussac, 1821), genetically sufficiently distant to be considered well-differenced species (Elejalde et al., 2008a), but with shells similar enough that non-experts have difficulty distinguishing between them (Ruiz Ruiz et al., 2006). At the same time, supposed species such as I. rositai de Fez, 1950 and I. cobosi Ibáñez et Alonso, 1978, with well-differentiated shells (pale brown, flattened, keeled and very ornamented) from the typical shells of the genus (more or least globose, frequently banded and with brown tones) resulted to be morphs of the same species (I. marmoratus; Elejalde et al., 2008a). This leads to a paradoxical situation: the genus Iberus displays a high conchological diversity, with contrasted flattened-keeled and globose-smooth shells (Cadevall & Orozco, 2016; Liétor, 2014; Ruiz Ruiz et al., 2006), but shell morphology alone does not help to differentiate species (Elejalde et al., 2005, 2008a, b). Similar findings have been reported for other land snails, in which among-populations morphological data do not match genetic distances (e.g. Haase & Bisenberger, 2003; Pfenninger & Magnin, 2001; Teshima et al., 2003). Similarly, the traditional use of genitalia morphology has also been proven ineffective for species delimitation in snails (Nantarat et al., 2019; Wilke et al., 2002). Therefore, the classification of snails exclusively based on morphological features can be misleading, as molecular techniques are necessary to properly delimit species (Pfenninger et al., 2006).
Hence, it is unclear how many species are included within the genus Iberus as well as their distributions and type morphologies. For this reason, to clarify the species diversity of this genus, we have performed a long-term study embracing the complete distribution area of the genus Iberus with the aim of unequivocally clarifying the diversity of species involved. Given that the genus includes cryptic species and foreseeing the occurrence of new cryptic species, we have tried to sample all possible populations (more than 1100 sampling points at the moment of which 281 are involved in the current study). Ulterior systematic genetic sequencing along with a comprehensive geographic mapping will allow us to delimit the actual species of the genus Iberus.
We consider that the branch of the evolutionary tree of the genus Iberus including the species I. ortizi and related species is solved in the present work. Here, we describe three new cryptic species of the genus Iberus: I. giennensis, I. axarciensis and I. antikarianus spp. nov., which, together with I. ortizi, constitute a consistent clade. One of the new species we describe here was already reported as a new species by Elejalde et al. (2008a) based on the genetic analysis of four specimens. Here, we confirm its phylogenetic position by adding new specimens and describe the species, delimiting its distribution. Elejalde et al. (2008a) detected a second new species in the phylogenetic tree supposed as I. loxanus (A. Schmidt, 1853) according to its shell morphology. However, this alleged I. loxanus was not clustered with the rest of I. loxanus and showed a divergence of 6% and 12% (16S and COI, respectively) with I. ortizi, the closest species. Given that this clade was originally defined by only one individual, we carried out intense field prospections to find new populations with similar haplotypes. Finally, a population matching the haplotype was found, which led us to describe the new species. Lastly, the third species we describe here was fortuity found during the field sampling routine when we sequenced individuals of a population suspected to be a new species close to I. ortizi.
To clarify the taxonomic identity of these three new species, besides the classical conchological description, we present a comprehensive dataset of morphometric analyses of hundreds of shells sampled in a large number of locations. Morphometric analyses allow us to make comparisons with other species of the genus Iberus that inhabit nearby geographic areas and present similar shells. Thus, the phylogenetic clarification of a large clade of the evolutionary tree of Iberus is addressed from an integrated perspective, using an intensive biogeographic characterisation, a phylogenetic study based on a significant number of samples and a morphometric study on a large number of shells that cover the broad phenotypic spectrum of the variability of these sister species.
Materials and methods
Field sampling
For two decades, we have performed systematics field sampling consisting of more than 1100 sampling points throughout Spain to determine the distribution of the species within the genus Iberus. Sampling points were determined according to (i) previous citations in specialised literature, (ii) the presence of karstic habitats or sedimentary lithology that provide adequate levels of calcium to form the shells (Fournié & Chétail, 1984) and (iii) the prior knowledge and field experience of the researchers. For each sampling point, we recorded the geographic coordinates and representative photographs of the habitat. For each Iberus species, a set of shells was collected, cleaned, photographed (with a Sony RX100 camera) in lateral, ventral and dorsal positions, and measured to obtain a number of morphometrics. From these sampling points, 82 correspond to the three species here described (I. giennensis sp. nov.: 48; I. axarciensis sp. nov.; 28; I. antikarianus sp. nov.: 6), plus 199 for the nearest species.
Morphometric measurements
Shell morphometric parameters were obtained following López-Alcántara et al. (1985). We measured with a digital calliper (accuracy 0.01 mm): the largest and the smallest diameter (Ø) of the shell, shell height and major and minor external Ø of the peristome. According to these data, we estimated the shell and peristome area, by considering that both the shell and the peristome may resemble an ellipse, applying the formula area = π × [(major Ø)/2] × [(minor Ø)/2]. On the basis of these measurements, we estimated the subsequent set of morphological ratios: shell height/minor Ø of the shell (H/W ratio, as an indicator of shell globosity, more globose shells having a higher ratio); major Ø of the shell/minor Ø of the shell (as an indicator of shell circularity, so that the closer this rate is to unity, the greater the degree of circularity of the shell); major external Ø of the peristome/minor external Ø of the peristome (as an indicator of peristome circularity); percentage of the total surface of the shell occupied by the peristome (calculated as (peristome area × 100)/shell area). All measurements were carried out by the same researcher (JL). The repeatability of all measurements (estimated according to Senar, 1999) was always > 0.99. We checked for outliers for the morphometrics measurements by using the Cleveland plot (following Zuur et al., 2010). No possible outlier was detected, but we detected eight individuals (of 1374) with extreme values for some variable, representing only 0.6% of measured shells. These extreme values, however, were not necessarily outliers, but only extreme values within the distribution of the data (Quinn & Keough, 2002). We also found individuals with odd shells (less than 0.5% of shells; Supplementary Fig. S1), which were not included in the morphometric analysis.
Statistical comparisons between morphometric measurements were carried out with ANOVA tests when the variables were homoscedastic and normally distributed, otherwise using the Kruskal-Wallis test. In addition, a principal components analysis was carried out to determine the overlap between the described species in the morphospace.
Phylogenetic analysis
Among all the specimens collected alive in the field, those from key locations were selected for genetic analysis. We consider as key locations for each species those that, being as far apart as possible within the distribution of the species, would make it possible to cover the entire distribution area in a representative manner, trying to embrace the maximal intraspecific genetic diversity. Once in the laboratory, the specimens were sacrificed by drowning and a tissue sample was extracted for molecular analyses. For this study, samples belonging to 14 individuals were stored in absolute ethanol and maintained at −20 °C. Genomic DNA was extracted using QIAGEN DNeasy Blood & Tissue Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. A fragment (~670 bp) of the mitochondrial cytochrome C oxidase subunit I (COI, standard barcoding fragment, with primers LCO and HCO; Folmer et al., 1994) was amplified by polymerase chain reaction (PCR). Following the standard protocols, negative controls were used in all PCR to detect possible contaminations. The obtained sequences (Genbank accession numbers: OR800623-36) were edited with Sequencher v5.4.6 (Gene Codes Corporation, Ann Arbor, MI, USA), and checked for potential contaminations using GenBank’s BLASTn search (Altschul et al., 1990). Sequences were aligned in Seaview v.4.2.11 (Gouy et al., 2010) under ClustalW2 (Larkin et al., 2007) default settings. The final alignment comprised 634 base pairs (bp) from 159 individuals including the outgroup (Otala lactea, Helicella sp. and Eobania vermiculata) (Supplementary Table S1). Uncorrected p-distances with partial deletion were computed in MEGA (Kumar et al. 2018). Phylogenetic relations of Iberus sequences were analysed for Bayesian inference (BI) analysis using MrBayes v3.2.6 (Ronquist & Huelsenbeck, 2003). The best model of sequence evolution (TPM2uf+I+G) was selected following the AIC using jModelTest v2.1.6 (Darriba et al., 2012). Two independent runs (each with four Markov chains for 5 × 107 generations) were performed. Trees and parameters were sampled every 1000 generations. Maximum likelihood (ML) searches were conducted in RAxML v7.0.4 (Silvestro & Michalak, 2012) using default settings and support was assessed by using 1000 bootstrapped replicates. The majority-rule consensus tree was estimated by combining results from duplicated analyses, after discarding 25% of the total samples as burn-in. All phylogenetic analyses were performed in the CIPRES platform (Miller et al., 2010). The consensus tree was visualised and rooted using FigTree v1.4.4 (Rambaut, 2018), and later prepared as a graphic with the software Inkscape v1.0.1 (http://www.inkscape.org).
Results
Phylogenetic position and genetic divergence
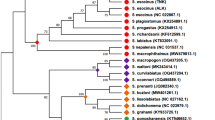
The results from the Bayesian inference and ML recovered the same three strongly supported (BI BPP = 1.00, ML BS = 100) monophyletic clades for the 14 new Iberus samples, but their positions differed between both analyses. Samples B1, B5, B6, B7, B8 (named Clade 1) were grouped with 4 sequences named Iberus sp. in Elejalde et al. (2008a) (Fig. 1). Sample U1 matched the sample I.loxanus01 in Elejalde et al. (2008a; Genbank accession EF440255) (named Clade 2). Lastly, samples U3, U4, U5, U7, AM1, AM3, AJ1, AJ2 formed the third clade (named Clade 3), which is a new clade formed only by our samples, with no close matches from Genbank. The BI recovered all clades sister to Iberus ortizi, again strongly supported. Clades 1 and 2 are sister clades with a BPP of 0.95, while clade 3 is a sister clade to the former with a weaker node supports (BPP = 0.84). The ML analyses recovered Clade 1 and Clade 2 as sister clades (BS = 60), these being sister to I. ortizi, but weakly supported (BS = 27). This group (Clade 1 + Clade 2 + I. ortizi) is sister to Clade 3 (BS = 86) (ML analyses are only reported and not shown). Future sequencing of additional markers might slightly change the phylogenetic positions between the three lineages here described.
At the left, a Bayesian tree inferred in MrBayes based on Iberus species COI sequence data. The branch examined in the present study is indicated in the tree in a different colour. At the right, an amplified vision of the branch analysed in the present study. In red, sequences of the Clade 1 (I. giennensis sp. nov.), in blue Clade 2 (I. antikarianus sp. nov.), and in green Clade 3 (I. axarciensis sp. nov.)
The p-uncorrected distances within clades were: 2.6% (within Clade 1), 3.8% (within Clade 2), 2.4% (within Clade 3), and 2.4% within I. ortizi. As expected, genetic divergences between clades were much higher, corroborating that these clades or lineages actually correspond to different species. The divergence between Clade 1 and Clade 2 was 12.2%, between Clade 1 and Clade 3, 12.5%, and between Clade 2 and 3 it was 12.6%. The genetic distances of these clades and I. ortizi are: I. ortizi – Clade 1, 11.8%; I. ortizi – Clade 2, 12,9%; and I. ortizi – Clade 3, 12.6% (Supplementary Table S2).
Therefore, as concluded from the phylogenetic analysis, three new species for the genus Iberus, phylogenetically closed, must be described. These species are named: I. giennensis (Clade 1), I. axarciensis (Clade 3) and I. antikarianus (Clade 2) spp. nov.
Iberus giennensis sp. nov.
Taxonomy
Phylum MOLLUSCA Cuvier, 1795
Class GASTROPODA Cuvier, 1795
Order STYLOMMATOPHORA A. Schmidt, 1855
Family HELICIDAE Rafinesque, 1815
Genus Iberus Montfort, 1810
Iberus giennensis sp. nov.
Etymology
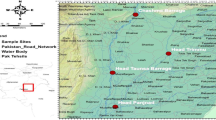
Twenty-seven of the 35 localities where I. giennensis sp. nov. was recorded belonged to the southern area of Jaen Province, most of them placed within the Sierra Sur mountain range (Fig. 2). Therefore, it seems appropriate to use the Latinism assignable to the name of the province where the species mainly inhabits to denominate this new species.
Map of the Iberian Peninsula (left image) and the right image is an inserted map showing the geographical distribution of I. giennensis (orange), I. axarciensis (purple), I. antikarianus spp. nov., (yellow), I. ortizi (green), I. angustatus (amber) and I. guiraoanus (turquoise). Points represent populations for which we have morphological data. Stars indicate those populations for which, moreover, we have genetical information. The bar for distances is in kilometres
Holotypes and paratypes
Figure 3 shows the photographs of the holotype and paratype shells assigned to I. giennensis sp. nov. Morphological measurements of holotype and paratype shells of I. giennensis sp. nov. are available in Table 1. The type locality for I. giennensis sp. nov. consists of calcareous slopes surrounding Valdearazo River Canyon, Sierra Sur, Valdepeñas de Jaen (Jaen Province, Spain), with the following coordinates: 37° 36′ 57′′ N, -3° 41′ 35′′ W.
Photographs of the holotype (H) and the 10 paratypes (P1 to P10) of I. giennensis sp. nov. Valle del río Valdearazo. Sierra Sur of Jaen. Valdepeñas de Jaen, Jaen (HOLOTYPE, 11/12/2006; ID code 22463-CG); Road to wind farm of Sierra del Trigo, Hoya del Salobral, Noalejo, Jaen (PARATYPE 1, 11/14/2021; ID code 22464-CG); close to N-432 in Castillo de Locubín, Sierra Sur, Jaen (PARATYPE 2, 01/16/2022; ID code 22465-CG); Cogollos de la Vega, Granada (PARATYPE, 3 03/20/2022; ID code 22466-CG); Ermita de la Verónica, Alcalá la Real, Sierra Sur, Jaen (PARATYPE 4, 09/05/2021); Cañada de las Hazadillas, Sierra Sur, Jaen (PARATYPE 5, 10/04/2021); close to Piñar castle, Piñar, Granada (PARATYPE 6, 01/30/2022); close to C/Calvario, la Rábita, Alcalá la Real, Sierra Sur, Jaen (PARATYPE 7, 01/16/2022); Pitillos Mount, near Quiebrajano reservoir, Sierra Sur, Jaen (PARATYPE 8, 09/21/2021); Barranco de los Corralones, Fuensanta de Martos, Jaen (PARATYPE 9, 10/28/2021) and Cañada de las Hazadillas, Sierra Sur, Jaen (PARATYPE 10, 02/16/2013). Holotype and paratypes 1–3 have been deposited in the Zoology Collections of the University of Granada (https://ccz.ugr.es/en); the paratypes 4-10 are placed in the private collection of Dr José Liétor Gallego. ID code refers to the identification codes in the Zoology Collections of the University of Granada. Photographs composition was carried out with Corel Photo Paint 12
Type shell description
Figure S2 in Supplementary Material shows a representative series of the conchological variability in I. giennensis sp. nov. Alive specimens of I. giennensis sp. nov. are represented in Fig. 4. Table S3 in Supplementary Material shows the average morphometric values for this species.
Iberus giennensis sp. nov. has a globose and not umbilicated shell, with 4–5 whorls of regular growth. The last whorl is convex, slightly compressed and more dilated than the others. The suture is simple and visible in all whorls. Protoshell has 1–1.5 turns with smooth and uniform light brown colour. The shell surface is irregularly striated giving a reticulum (except in the protoshell), with prominent radial ribs that are distributed in a non-regular pattern between less pronounced transverse striations. Shell aperture is large, oval-semilunar, wider than high, having a fine not reflected peristome (somewhat sharp in the palatal wall). The peristome shows a slight thickening in the area of the columellar wall, close to the umbilicus. There is no callus on the parietal edge and the umbilicus area. Thickening and whitish tone typical in the umbilicus area of other species of the genus Iberus are absent in I. giennensis sp. nov. Sometimes, the umbilicus area exhibits a slight depression.
The colour of the shell in the first three whorls is light brown and off-white (bone colour) in the rest. The body whorl of the shell is longitudinally crossed by five dark brown bands, of which the top three are frequently discontinuous. A minor percentage of shells may have upper continuous bands. The two lower bands are wider, continuous or discontinuous, being the top one between two and four times wider than the one at the bottom. The area over the two principal bands of the body whorl may exhibit a slight-spotted pattern of white/light cream tones that turns dense in some cases. The colour of the lip is off-white, although some specimens are pink, even intense fuchsia.
Three predominant morphotypes are distinguished in I. giennensis sp. nov. based on their band patterns (Fig. 5). (i) Morphotype 1: consisting of two continuous dark brown lateral bands in the body whorl, being the upper one between two and four times wider than the lower one (only in a very few cases, both bands become similar in width). (ii) Morphotype 2: lateral bands in the body whorl turn intermittent or diffuse, sometimes with lighter or even pale tones. (iii) Morphotype 3 (the rarest one): the same as morphotype 1 but with bands which continue in the upper whorls until reaching the protoconch. Morphotypes 1, 2 and 3 represent 60%, 36% and 4% of the shells sampled, respectively. Further research will determine which pedoclimatic and ecological factors influence the relative abundance of each of these morphotypes to establish if they might be considered as ecotypes.
Morphotypes of I. giennensis sp. nov. 1. Continuous bands in body whorl. 2. Discontinuous bands in the body whorl. 3. Continuous bands in the whole shell. All shells sampled in Castillo Locubín, Sierra Sur (Jaen Province). Photos mounted as in Fig. 3
Habitat and distribution
The ecological niche of I. giennensis sp. nov. consists of rock formations on a calcareous-based lithology between 574 and 1443 m in altitude in Southern Jaen and Northern Granada Provinces (south Spain; Figs. 2 and 6). Although it is most common to find I. giennensis sp. nov. inside the cracks and cavities and under the stones of limestone pavements and rocky ridges, it also inhabits natural or planted Mediterranean scrublands and forests, being found under the leaf litter layer, even pine needles.
Some habitats of I. giennensis sp. nov. 1. Sierra Pelada, Íllora, Granada; 2. Hoya del Salobral, Noalejo, Jaen; 3. Río Susana, Valdepeñas de Jaen; 4. Sierra del Trigo, Noalejo, Jaen; 5. Cogollos de la Vega, Granada; 6. Sierra de Ahillos Alcaudete, Jaen; 7. Arroyo del Rigüelo, Fuensanta de Martos, Jaen; 8. Barranco de los Correlones, Fuensanta de Martos, Jaen; 9. Cerro Santa Merced, Montillana, Granada; 10. Cerro del Hoyo, Valdepeñas de Jaen; 11. Puerto de Navaleón, Noalejo, Jaen; 12. Cañada de las Hazadillas, Parque Periurbano Monte la Sierra, Jaen
Conservation status
Iberus giennensis sp. nov. seems to present healthy populations based on the medium–high density of specimens found in the large number of locations sampled. In fact, I. giennensis sp. nov. has a wide potential distribution area, estimated at approximately 2000 km2, as it occupies calcareous mountain massifs that provide a continuity of potential habitats. However, I. giennensis sp. nov. presents a singular population in the Sierra Arana (Granada Province), separated from the main population nucleus at the north for a zone occupied by I. angustatus (Rossmässler, 1854). This population is probably isolated, deserving additional conservation effort.
Iberus axarciensis sp. nov.
Taxonomy
Phylum MOLLUSCA Cuvier, 1795
Class GASTROPODA Cuvier, 1795
Order STYLOMMATOPHORA A. Schmidt, 1855
Family HELICIDAE Rafinesque, 1815
Genus Iberus Montfort, 1810
Iberus axarciensis sp. nov.
Etymology
The name of I. axarciensis sp. nov. refers to the Axarquía, a region of the province of Malaga (South Spain), where most of the localities of I. axarciensis sp. nov. are located (Fig. 2).
Holotypes and paratypes
Figure 7 shows the photographs of the holotype and paratype shells assigned to I. axarciensis sp. nov. Morphological measurements of holotype and paratype shells of I. axarciensis sp. nov. in Table 2. The type locality for I. axarciensis sp. nov. is assigned to the surroundings of Alfarnate, Malaga Province (Spain), with the following coordinates: 37° 00′ 15′′ N, -4° 16′ 31′′ W.
Photographs of the holotype (H) and the 10 paratypes (P1 to P10) of I. axarciensis sp. nov. Close to the intersection between A-4152 and MA-155, Alfarnate, Malaga (HOLOTYPE, 11/12/2022; ID code 22459-CG); Camino del Tallón, Alfarnatejo, Malaga (PARATYPE 1, 08/10/2022; ID code 22460-CG); Peña Negra, Villanueva de Cauche, Antequera, Malaga (PARATYPE 2, 11/28/2021; ID code 22461-CG); Eastern slope of Puerto del Sol, Periana, Malaga (PARATYPES 3 and 4, 10/15/2022; ID code 22462-CG for paratype 3); Periana, Malaga (PARATYPE 5, 11/19/2022); Cortijo la Parrilla, Fuente Camacho, Loja, Granada (PARATYPES 6 and 7, 10/08/2022); Close to A-4152, Puerto de los Alazores, Sierra de San Jorge, Loja, Granada (PARATYPE 8, 10/08/2022); Camino del Tallón, Alfarnatejo, Malaga (PARATYPE 9, 10/08/2022) and Los Sabucos, Sierra Gorda, El Almendral, Zafarraya, Granada (PARATYPE 10, 11/26/2022). Holotype and paratypes 1–3 have been deposited in the Zoology Collections of the University of Granada (https://ccz.ugr.es/en); the paratypes 4–10 are placed in the private collection of PhD José Liétor Gallego. ID code refers to the identification codes in the Zoology Collections of the University of Granada. Photographs composition was carried out with Corel Photo Paint 12
Type shell description
Figure S3 in Supplementary Material shows a representative series of the conchological variability in I. axarciensis sp. nov. Alive specimens of I. axarciensis sp. nov. are represented in Fig. 8. Table S4 in Supplementary Material shows the average morphometric values for I. axarciensis sp. nov.
Some alive specimens of I. antikarianus (1) and I. axarciensis (2-12) spp. nov. photographed in situ. 1. Peña de los Enamorados, Antequera, Malaga; 2–4. Different specimens in Cortijo la Parrilla, Fuente Camacho, Loja, Granada; 5. Arroyo de las Mozas, Fuente Camacho, Loja, Granada; 6. Periana, Malaga; 7. Alfarnate, Málaga; 8–10. Different specimens in Puerto de los Alazores, Alfarnate, Malaga; 11. Puerto del Sol, Periana, Malaga; 12. El Almendral, Zafarraya, Granada
Iberus axarciensis sp. nov. has a not umbilicated globose shell with 4–5 whorls of regular growth, the last of which is convex and slightly compressed, being more dilated than the others. Shells have simple and visible sutures in all whorls. Protoshell shows 1–2 smooth whorls with uniform light brown colour. Contrastingly, a fine and homogeneous transverse striation can be seen in the rest of the whorls. As a result of the radial striation being mixed with the longitudinal one, a fine and regular reticulation appears. Shell aperture is large, oval-semilunar, wider than high, having a fine variable peristome that sometimes is sharp but, in some localities, becomes slightly expanded. The peristome shows a slight thickening in the columellar wall, close to the umbilicus. There is no callus either on the parietal edge or in the umbilicus area. Thickening and whitish tones typical in the umbilicus area of other species of the genus Iberus are absent in I. axarciensis sp. nov. Sometimes, the umbilicus area exhibits a slight depression.
The colour of the shell in the first three whorls is light or pale brown, whilst the rest is off-white. Nevertheless, some populations show uniform off-white colour for all whorls. The shell body whorl is crossed by five dark brown bands, the two upper ones usually discontinuous. Nevertheless, a minor percentage of shells may have upper continuous bands. Regarding the three lower bands, the two at the bottom use to be wide whilst the upper one may be very fine in some cases. All these three bands can be continuous or discontinuous, even diffused sometimes. Considering the two lower main bands, the top one may become between 0.5 and 3 times wider than the one at the bottom. The area over the three principal bands of the body whorl may exhibit a slight spotted pattern of white/light cream tones that turns dense in some cases. Lips of all the specimens sampled were off-white (never light or dark pink).
Four morphotypes can be assigned to I. axarciensis sp. nov. (Fig. 9), of which the first two are clearly predominant: (i) Morphotype 1: flattened specimens with two continuous dark brown lateral bands, the upper one between two and four times wider than the lower one, although shells of some populations may show fine bands of similar width. (ii) Morphotype 2: the same as morphotype 1 but with lateral bands turning intermittent or diffuse, sometimes with lighter or even pale tones. (iii) Morphotype 3: globose specimens with two continuous dark brown lateral bands (the upper one, at most, twice as wide as the bottom one). Nevertheless, both bands may have the same width on some occasions. (iv) Morphotype 4: the same as morphotype 3 but with lateral bands turning intermittent or diffuse, sometimes with lighter or even pale tones.
Morphotypes of I. axarciensis sp. nov. 1. Flattened continuous bands (Periana, Malaga). 2. Flattened discontinuous bands (Puerto de los Alazores, San Jorge Mountains, Alfarnate, Malaga). 3. Globose continuous bands (Puerto del Sol, Periana, Malaga). 4. Globose discontinuous bands (this is the only good shell of this morphotype the authors were able to find; Puerto del Sol, Periana, Malaga). Photos composition mounted as in Fig. 3
Morphometric parameters showed significant differences between the flattened and globose morphotypes (Table 3). Flattened morphotypes are larger, less tall and have a less circular shell and peristome (ratios between major and minor diameters farther than 1) than the globose morphotypes. Thus, the flattened morphotypes turned out to be significantly less conical. Although the peristome of the flattened morphotype is larger, there are no significant differences in the proportion of the total shell surface occupied by peristomes between both morphotypes. Of the whole set of shells of I. axarciensis sp. nov. checked, 30% correspond to the globose morphotype and the remaining 70% to the flattened one. Specimens with continuous bands accounted for 99% of the globose morphotype while they decreased to 57.8% in the flattened morphotype.
Habitat and distribution
The ecological niche of I. axarciensis sp. nov. consists of karstic limestone areas between 705 and 1318 m of altitude in the Northeast of Malaga and the Western end of Granada Provinces (south Spain; Figs. 2 and 10). Although it is most common to find I. axarciensis sp. nov. inside the cracks and cavities and under the stones of limestone pavements and rocky ridges, it also inhabits natural Mediterranean scrublands, even marginal and degraded areas near crops and ditches.
Some habitats of I. antikarianus (1–2) and I. axarciensis (3–12) spp. nov. 1. Surroundings of Archidona Castle, Malaga; 2. Peña de los Enamorados, Antequera, Malaga; 3. Alfarnate, Malaga; 4. Arroyo del Morrón, Alfarnatejo, Malaga; 5. Camino del Tallón, Alfarnatejo, Malaga; 6. Puerto de los Alazores, Alfarnate, Malaga; 7. Los Sabucos, Sierra Gorda, El Almendral, Zafarraya, Granada; 8. Periana, Malaga; 9. Rincón de los Reinas, Zafarraya, Granada; 10. Fuente de los 100 Caños, Villanueva del Trabuco, Malaga; 11.
Conservation status
Iberus axarciensis sp. nov. have wide potential distribution areas of approximately 550 km2, occupying calcareous mountains that provide a continuity of potential habitats. No conservation concerns have been identified for this species.
Iberus antikarianus sp. nov.
Taxonomy
Phylum MOLLUSCA Cuvier, 1795
Class GASTROPODA Cuvier, 1795
Order STYLOMMATOPHORA A. Schmidt, 1855
Family HELICIDAE Rafinesque, 1815
Genus Iberus Montfort, 1810
Iberus antikarianus sp. nov.
Etymology
The name of I. antikarianus sp. nov. refers to the town of Antequera (Malaga Province), whose name during Roman times was “Antikaria”. Only three localities for I. antikarianus sp. nov. have been found (Fig. 2), one of them placed in Peña de los Enamorados (Antequera) being the most relevant in terms of abundance and diversity of specimens.
Holotypes and paratypes
Figure 11 shows the photographs of the holotype and paratype shells assigned to I. antikarianus sp. nov. Morphological measurements of holotype and paratype shells of I. antikarianus sp. nov. are available in Table 4. The type locality for I. antikarianus sp. nov. consists of calcareous rocks of limestone areas with Mediterranean scrublands. The type locality for I. antikarianus sp. nov. is assigned to the Southern end of Peña de los Enamorados, Antequera, Malaga Province (Spain) with the following coordinates: 37° 03′ 35′′ N, −4° 29′ 29′′ W.
Photographs of the holotype (H) and the 10 paratypes (P1 to P10) of I. antikarianus sp. nov. Southern area of Peña de los Enamorados, Antequera, (HOLOTYPE and PARATYPES 4, 5 and 7, 11/28/2021; ID code 22455-CG); Northern area of Peña de los Enamorados, Antequera, (PARATYPES 1, 2, 3 and 6; 07/30/2022; ID codes 22456-CG to 22458-CG, respectively for paratypes 1, 2 and 3); Surroundings of Archidona Castle, Malaga (PARATYPES 8, 9 and 10; 11/18/2022). Holotype and paratypes 1–3 have been deposited in the Zoology Collections of the University of Granada (https://ccz.ugr.es/en); the paratypes 4–10 are placed in the private collection of PhD José Liétor Gallego. ID code refers to the identification codes in the Zoology Collections of the University of Granada. Photographs composition was carried out with Corel Photo Paint 12
Type shell description
Figure S4 in Supplementary Material shows a representative series of the conchological variability in I. antikarianus sp. nov. Alive specimens of this species are represented in Fig. 8. Table S5 in Supplementary Material shows the average morphometric values for this species.
No remarkable conchological differences can be established between I. axarciensis and I. antikarianus spp. nov. Shape, structure and ornamentation in both species are considerably similar, although differences may be found at the population level. As I. axarciensis sp. nov., I. antikarianus sp. nov. has a not umbilicated globose shell with 4–5 whorls of regular growth, the last of which is convex and slightly compressed, being more dilated than the others. Shells have simple and visible sutures in all whorls. Protoshell shows 1–2 smooth whorls with uniform light brown colour. Contrastingly, a fine and homogeneous transverse striation can be seen in the rest of the whorls. As a result of the radial striation being mixed with the longitudinal one, a fine and regular reticulation appears. Shell aperture is large, oval-semilunar, wider than high, having a fine variable peristome that sometimes is sharp but, in some localities, becomes slightly expanded. The peristome shows a slight thickening in the columellar wall, close to the umbilicus. There is no callus either on the parietal edge or in the umbilicus area. Thickening and whitish tones typical in the umbilicus area of other species of the genus Iberus are absent in I. antikarianus sp. nov. Sometimes, the umbilicus area exhibits a slight depression.
The colour of the shell in the first three whorls is light or pale brown, whilst the rest is off-white. Nevertheless, some populations show uniform off-white colour for all whorls. The shell body whorl is crossed by five dark brown bands, the two upper ones usually discontinuous. Nevertheless, a minor percentage of shells may have upper continuous bands. Regarding the three lower bands, the two at the bottom use to be wide whilst the upper one may be very fine in some cases. All these three bands can be continuous or discontinuous, even diffused sometimes. Considering the two lower main bands, the top one may become between 0.5 and 3 times wider than the one at the bottom. The area over the three principal bands of the body whorl may exhibit a slight spotted pattern of white/light cream tones, that turns dense in some cases. Lips of all the specimens sampled were off-white (never light or dark pink).
In I. antikarianus sp. nov., there are morphotypes based on the pattern of bands: Morphotype 1: specimens with two continuous dark brown lateral bands, the upper one between two and four times wider than the lower one, although shells of some populations may show fine bands of similar width. Morphotype 2: the same as morphotype 1 but with lateral bands turning intermittent or diffuse, sometimes with lighter or even pale tones.
Habitat and distribution
Iberus antikarianus sp. nov. has been only found in karstic areas between 481 and 905 m in altitude in a restricted area of a few mountains in Malaga province (Figs. 2 and 10). Again, live snails were always associated with limestone (in cracks, cavities, under stones). The potential distribution area of this species should be considered small and fragmented due to the amount of natural (rivers) and human barriers (orchard plantations and roads) surrounding it.
Conservation status
Unlike the previous two taxa, I. antikarianus sp. nov. presents a very small potential distribution, barely 50 km2 when considering that the only three populations found inhabit isolated mountains separated from each other by cereal crops and olive groves as well as by a highway. We therefore consider that I. antikarianus sp. nov. should be one of the species of the genus Iberus candidate to be assigned an eventual conservation category.
Differences between shells of I. giennensis, I. axarciensis and I. antikarianus spp. nov.
Shells of I. giennensis sp. nov. and the pool constituted by I. axarciensis and I. antikarianus spp. nov. are certainly similar. Still, some distinguishing features may be listed; (i) peristome edge is slightly less projected (on average) in I. giennensis sp. nov. than in the other two species, which included some localities where peristome edge tends to spread. (ii) I. giennensis sp. nov. typically presents two main lateral bands in the body whorl, while the other two species typically present three bands in most of the specimens. (iii) Shell mottling is more intense in I. giennensis sp. nov. By contrast, shell mottling is sparser in the other two species, being absent in the individuals of some populations. (iv) Pinkish, even fuchsia lip is frequent in alive and fresh specimens of I. giennensis sp. nov., but this feature does not occur in I. axarciensis and I. antikarianus spp. nov. (v) Shell surface of I. giennensis sp. nov. shows non-periodical radial cords resulting in an irregular radial striation. Contrastingly, shells of I. axarciensis and I. antikarianus spp. nov. show a fine and homogeneous radial striation mixed with the longitudinal one, producing a fine regular reticulation, much finer than that found in I. giennensis sp. nov.
In addition, statistically, the shells of I. axarciensis sp. nov. exceeded on average in size (in wide, height and total area) those of I. antikarianus sp. nov., which, at the same time, were on average larger than those of I. giennensis sp. nov. (Table 5). However, the shape of the shells differed according to the species. The highest average H/W ratio was measured in I. giennensis sp. nov., being significantly higher than that in I. axarciensis and I. antikarianus spp. nov. (which showed a statistically similar H/W ratio, on average; Table 5). This indicates that shells in I. giennensis sp. nov. tended to be less flattened than shells in the other two species. The highest average ratio between the maximal and the minimal diameters (circularity) was found in I. antikarianus sp. nov., being significantly higher than that in I. axarciensis sp. nov. which, in turn, had a mean circularity ratio significantly higher than I. giennensis sp. nov. (Table 5). Considering that the closer this ratio to 1, the more circular the shell, I. giennensis sp. nov. had, on average, the most circular shell, while shells of I. antikarianus sp. nov. were the most oval-shaped shells, occupying those of I. axarciensis sp. nov. an intermediate position.
The form of the peristome also differed, on average, between the three species. Peristome was, on average, larger in I. axarciensis sp. nov., followed by I. antikarianus sp. nov., and the smallest in I. giennensis sp. nov. (Table 5). This coincides with the differences in the shell size. However, the mean area of peristome regarding the total shell area was the highest in I. antikarianus sp. nov., showing that this species possesses a peristome proportionally larger than the other two species. In addition, the peristome of I. antikarianus sp. nov. was more oval-shaped than that of I. axarciensis and I. giennensis spp. nov., which presented more rounded peristomes.
A PCA provided a first factor (PC1) accounting for 55.94% of the variance in shell morphology, which included shell area and percentage of shell surface that is occupied by the peristome. PC1 may be interpreted as a gradient of shell size and the relative size of the peristome. The second factor (PC2) explained 32.29% of the variance and included the ratio between shell height and major shell diameter, that is, the index of globosity. On one hand, the PC1 grouped populations of I. axarciensis and I. antikarianus spp. nov. as the largest with I. giennensis sp. nov. and I. ortizi having intermediate and small shells, respectively (Fig. 12). On the other hand, according to PC2, I. axarciensis, I. antikarianus spp. nov. and I. ortizi presented flatter shells than I. giennensis sp. nov., whose average shell was the more globose of the clade. However, the four species still showed considerable overlap in their morphology (Fig. 12). This variation did not show either apparent geographical pattern, except for I. axarciencis sp. nov., whose oriental populations were flattener globose than the occidental ones (Fig. 13).
Distribution of I. giennensis sp. nov. (23 localities), I. axarciensis sp. nov. (18 localities), I. antikarianus sp. nov. (2 localities) and I. ortizi (21 localities) in the bi-dimensional space generated by the two first principal components of a PCA analysis. Each point in the graph represents a single sampling locality. Coordinates of centroids for each species have been calculated as the average X and Y coordinates of the points included in the corresponding clouds
Geographical distribution of the scores for the first (A) and second (B) principal components shown in Fig. 12. A colour sequence from light yellow to red has been used, corresponding to the 5 quintiles of the score ranges produced by the two first axes of the PCA. Notice that not all the sampling localities in Fig. 2 are represented here because sufficient conchological material was not available from several populations to carry out the PCA
Morphometric comparison among I. giennensis, I. axarciensis and I. antikarianus spp. nov. and other Iberus taxa geographically close with similar shells
We analysed statistically the shell differences between the three new species described here and I. ortizi, I. angustatus and I. marmoratus loxanus, three taxa belonging to the Iberus genus which are both geographically and conchologically close (Fig. 14). The comparison shows statistical differences in the average shell morphology between them. Shells of I. giennensis sp. nov. were, on average, wider, taller and more globose than those of I. ortizi, I. angustatus and I. marmoratus loxanus (Table 6). However, the shells of I. ortizi and I. angustatus were slightly more circular than that of I. giennensis sp. nov. (ratio between major and minor shells diameters closer to 1 in the two first), but I. marmoratus loxanus tended to be less globose than I. giennensis sp. nov. (Table 6). Regarding the peristome, that of I. giennensis sp. nov. had on average a greater relative surface area with respect to the total surface area of the shell than peristomes of I. ortizi and I. angustatus, but differences with I. marmoratus loxanus were not found (Table 6). However, I. giennensis sp. nov. showed the most circular peristome in comparison with the other three close species (Table 6).
Representative shells (banded morphotypes) of Iberus species which have been compared in this study. 1. I. giennensis sp. nov. from Fuensanta de Martos (Jaen); 2. I. axarciensis sp. nov. from Fuente Camacho, Loja (Granada); 3. I. antikarianus sp. nov. from Peña de los Enamorados, Antequera (Malaga); 4. I. angustatus from Santa Catalina Mountain (Jaen); 5. I. marmoratus loxanus from Moraleda de Zafayona (Granada); 6. I. ortizi from Zuheros (Córdoba). For comparative purposes, all shells selected have similar maximum diameters, around 20 mm. Photos mounted as in Fig. 3
The shells of I. axarciensis and I. antikarianus spp. nov. were on average wider and taller than those of I. ortizi, I. angustatus and I. marmoratus loxanus (Table 6). However, shell shape varied among species. Meanwhile, I. axarciensis and I. antikarianus spp. nov. shells were less globose than those of I. ortizi but showed a significantly higher degree of globosity than I. angustatus and I. marmoratus loxanus shells (Table 6). Regarding circularity, I. axarciensis and I. antikarianus spp. nov. were on average less circular on average than those of I. ortizi and I. angustatus but showed a similar circularity to I. marmoratus loxanus (Table 6). Peristomes of I. axarciensis and I. antikarianus spp. nov. manifested a greater mean relative surface area with respect to the total surface area of the shell and were more circular than those of I. ortizi, I. angustatus and I. marmoratus loxanus (Table 6).
Two other shell features showed clear differences between the six species compared: the proportion of shells showing contrasting banding patterns (continuous versus discontinuous bands) and differential degrees of umbilicus opening (closed versus somewhat open) (Table 7). Continuous bands are frequent in populations of I. giennensis sp. nov., I. axarciensis sp. nov., I. ortizi and I. angustatus, but less than 50% of individuals in the populations of I. antikarianus spp. nov. and I. marmoratus loxanus exhibited continuous bands. Besides, a closed umbilicus was the norm in most of the species, except for I. angustatus, in which only 37% of individuals present a completely closed one (Table 7).
Discussion
In this study, we describe three new species of the genus Iberus: I. giennensis, I. axarciensis and I. antikarianus spp. nov. Morphologically, the three described species show overlap in the form of their shells, although with slight differences at the population level. Therefore, they should be considered as cryptic species. Furthermore, these three species show morphologies very similar to other close species with nearby distributions: I. ortizi, I. angustatus and I. marmoratus loxanus. All these species have to be identified by genetic distances rather than morphological features. Most of them present allopatric distributions, suggesting that they evolved by allopatric speciation as a consequence of evolutionary isolation. Besides leading us to describe some new species, our findings show insights into the evolution of land snails in southeastern Spain, with implications for their conservation.
One of the here described species, I. giennensis sp. nov., was identified as a new species in the phylogenetic analysis by Elejalde et al. (2008a), based on the sequencing of four individuals. Here, we have increased the sample size with five additional individuals, delimiting more precisely the distribution range for the species, its habitat and its morphology (based on the measurements of 313 individuals). Nevertheless, the description of I. giennensis sp. nov. cannot be addressed without considering the taxon I. alcarazanus (Guirao in Rossmässler, 1854). I. alcarazanus was originally described by Rosmässler (1854) as Helix alcarazana. Although he considered this species to be distributed in the Sierra de Alcaraz (Albacete province, Spain), I. alcarazanus is not present in this mountain, being its type locality sited in Castellón de la Plana (east Spain; Martínez-Ortí & Robles, 2012). Moreover, posterior authors considered as I. alcarazanus specimens from southeastern Spain, 500 km far away from its type locality (García San Nicolás, 1957; Arrébola, 1995; Ruiz Ruiz et al., 2006; Liétor et al., 2014). Consequently, I. alcarazanus, due to its high conchological variability (Liétor et al., 2014), which makes it difficult to be differentiated from other taxa (Ruiz Ruiz et al., 2006), and to be considered as an invalid taxon or a junior synonym of Iberus alonensis (Férussac, 1821) (Martínez-Ortí & Robles, 2012), has not been take account in publications on the taxonomy of the genus Iberus (Cadevall & Orozco, 2016; Elejalde et al., 2008a). Given such a degree of uncertainty, we consider that it is pertinent to describe a new species called I. giennensis sp. nov. to designate the species of the genus Iberus with their own and well-differentiated characters that inhabits the southwest of the province of Jaen and the northwest of the province of Granada, and which matches with the species that was considered as I. alcarazanus in previous publications (García San Nicolás, 1957; Arrébola, 1995; Ruiz Ruiz et al., 2006; Liétor et al., 2014).
Another species described here, I. antikarianus sp. nov., was detected in the phylogenetic analysis by Elejalde et al. (2008a) based on the sequencing of a single individual initially considered as I. loxanus according to its morphology. The genetic distance between this individual and I. ortizi (the nearest taxon) was sufficient to consider it as a new species, and Elejalde et al. (2008a, p. 196) claimed that “[m]ore intensive sampling in the east of Malaga could provide more information about the phylogenetic relationships of this unique population”. Following their recommendation, we carried out an intense field sampling programme to obtain more individuals with similar haplotype and define the distribution range of this possible species. A new specimen was sequenced, confirming the existence of this species, whose distribution was defined. In addition, the morphology of this species has been characterised through the morphological analysis of 301 individuals. The scarce distribution of this species, restricted to no more than 300 km2 (much less if cultivated areas and population centres interspersed in their potential area are ruled out), could imply a concern in terms of conservation.
The third species described here, I. axarciensis sp. nov. was identified on the basis of genetic analysis. This species remained unnoticed until now as a consequence of its limited distribution and the similarity of its shell with other close and nearby Iberus species. No specimens of this species were considered in the study by Elejalde et al. (2008a), highlighting the importance of developing a research plan to achieve a comprehensive phylogeny and assess the systematics of the Iberus genus. The main criterium that has allowed researchers to describe new Iberus species in recent decades has been based on conchological data. However, it is likely that the genus Iberus hides a number of cryptic species that have not been identified based on the shell characteristics. Therefore, it seems necessary to conduct a systematic prospection and ulterior genetic sequencing of every population of Iberus sp. to assess the presence of cryptic species.
With this phylogenetic clade and their distribution at hand, we can postulate on some evolutionary processes of the species conforming to this clade. The two more ancestral species, I. angustatus and I guiraoanus (L. Pfeiffer, 1853), inhabiting Sierra Mágina and Sierra de Cazorla, Segura y las Villas, respectively, are located further north of all other species within the clade. Thus, this distribution suggests a possible expansion of species towards the southwest. Iberus ortizi sister clade relationship to all three new species described suggests conditions in which isolated ancestral I. ortizi populations eventually favoured speciation processes in the region. However, the lack of timing of the phylogeny limits the accuracy of understanding such processes. Today, I. giennensis sp. nov. coexists with I. angustatus in the eastern area of its distribution, without any apparent geographical barrier separating them. This sympatric distribution, as well as the presence of possible hybrids (personal observations) between the two species, merits further investigation.
Lastly, our findings not only have evolutionary and taxonomic implications. The three species considered here show very limited distributions, especially I. antikarianus sp. nov. This probably will involve conservation concerns. Although we have delimited the distribution of these species, population size and trends, as well as their threats, remain unknown. Iberus gualtieranus is the most studied Iberus species (Moreno-Rueda, 2011) and the only one currently included in the Spanish red list of invertebrates (Verdú et al., 2011). Iberus gualtieranus is considered as “Endangered” by the IUCN (Arrébola, 2011a). Still, the distribution of I. gualtieranus, with several isolated populations, is similar to or even larger than those of I. axarciensis and I. antikarianus spp. nov. In fact, I. ortizi, with a very small distribution range, similar to that of I. axarciensis and I. antikarianus spp., is considered as “Vulnerable” by the IUCN (Arrébola, 2011b). These discrepancies probably relate to the scarce information for I. ortizi in comparison with I. gualtieranus. Detailed studies of population size, structure and trends (as those done for I. gualtieranus; Moreno-Rueda & Pizarro, 2007) should be carried out for I. ortizi and the three new species described in this study to understand the conservation concerns of these endemics. Description of new endemic cryptic species and their distribution ranges will likely reveal a geographic mosaic of several morphologically similar species with restricted distributions with important conservation concerns. Many of these species, given their reduced distribution range, will probably gather the conditions needed to be catalogued as “Vulnerable” or even “Endangered”.
Data availability
All data generated or analysed during this study are included in this published article (Supporting Information).
References
Abellán, P., & Svenning, J.-C. (2014). Refugia within refugia - Patterns in endemism and genetic divergence are linked to Late Quaternary climate stability in the Iberian Peninsula. Biological Journal of the Linnean Society, 113, 13–28.
Altschul, S. F., Gish, W., Miller, W., Myers, E. W., & Lipman, D. J. (1990). Basic local alignment search tool. Journal of Molecular Biology, 215, 403–410.
Arrébola, J. R. (1995). Caracoles terrestres (Gastropoda, Stylommatophora) de Andalucía, con especial referencia a las provincias de Sevilla y Cádiz. PhD. Thesis. Seville: Universidad de Sevilla.
Arrébola, J. (2011a). Iberus gualtieranus. The IUCN Red List of Threatened Species 2011, e.T184948A8338429. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T184948A8338429.en. Accessed on 17 Mar 2023.
Arrébola, J. (2011b). Iberus ortizi. The IUCN Red List of Threatened Species 2011, e.T184954A8339647. https://doi.org/10.2305/IUCN.UK.2011-1.RLTS.T184954A8339647.en. Accessed on 17 Mar 2023.
Cadevall, J., & Orozco, A. (2016). Caracoles y babosas de la Península Ibérica y Baleares. Omega.
Cuvier, G. (1795) Second Mémoire sur l'organisation et les rapports des animaux à sang blanc, dans lequel on traite de la structure des Mollusques et de leur division en ordre, lu à la société d'Histoire Naturelle de Paris, le 11 prairial an troisième [30 May 1795]. Magazin Encyclopédique, ou Journal des Sciences, des Lettres et des Arts, 1795 [1. année] 2: 433–449. http://www.biodiversitylibrary.org/page/6736775
Darriba, D., Taboada, G., Doallo, R., & Posada, D. (2012). jModelTest 2: More models, new heuristics and parallel computing. Nature Methods, 9, 772. https://doi.org/10.1038/nmeth.2109
de Fez, S. (1950). Iberus rositai. Nueva especie del grupo de la H. gualtierana. Boletín de la Real Sociedad Española de Historia Natural, 48, 159–162.
Elejalde, M. A., Madeira, M. J., Arrébola, J. R., Muñoz, B., & Gómez-Moliner, B. J. (2008a). Molecular phylogeny, taxonomy and evolution of the land snail genus Iberus (Pulmonata: Helicidae). Journal of Zoological Systematics and Evolutionary Research, 46, 193–202.
Elejalde, M. A., Madeira, M. J., Muñoz, B., Arrébola, J. R., & Gómez-Moliner, B. J. (2008b). Mitochondrial DNA diversity and taxa delineation in the land snails of the Iberus gualtieranus (Pulmonata, Helicidae) complex. Zoological Journal of the Linnean Society, 154, 722–737.
Elejalde, M. A., Muñoz, B., Arrebola, J. R., & Gómez-Moliner, B. J. (2005). Phylogenetic relationships of Iberus gualtieranus and Iberus alonensis (Gastropoda: Helicidae) based on partial mitochondrial 16S RNA and COI gene sequences. Journal of Molluscan Studies, 71, 349–355.
Folmer, O., Black, M., Hoeh, W., Lutz, R., & Vrijenhoek, R. (1994). DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Molecular Marine Biology and Biotechnology, 3, 294299. https://doi.org/10.1093/bioinformatics/17.8.754
Fournié, J., & Chétail, M. (1984). Calcium dynamics in land gastropods. American Zoologist, 24, 857–870.
Gouy, M., Guindon, S., & Gascuel, O. (2010). SeaView version 4: A multiplatform graphical user interface for sequence alignment and phylogenetic tree building. Molecular Biology and Evolution, 27, 221–224.
Haase, M., & Bisenberger, A. (2003). Allozymic differentiation in the land snail Arianta arbustorum (Stylommatophora, Helicidae). Journal of Zoological Systematics and Evolutionary Research, 41, 175–185.
Husemann, M., Schmitt, T., Zachos, F. E., Ulrich, W., & Habel, J. C. (2014). Palaearctic biogeography revisited: Evidence for the existence of a North African refugium for Western Palaearctic biota. Journal of Biogeography, 41, 81–94.
Ibáñez, M., & Alonso, M. R. (1978). El género Iberus Montfort, 1810. 2. Iberus cobosi n.sp. Archiv für Molluskenkunde, 108, 193–200.
Kumar, S., Stecher, G., Li, M., Knyaz, C., & Tamura, K. (2018). MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547–1549.
Larkin, M. A., Blackshields, G., Brown, N. P., Chenna, R., Mcgettigan, P. A., Mcwilliam, H., Valentin, F., Wallace, I. M., Wilm, A., Lopez, R., Thompson, J. D., Gibson, T. J., & Higgins, D. G. (2007). Clustal W and clustal X version 2.0. Bioinformatics, 23, 2947–2948. https://doi.org/10.1093/bioinformatics/btm404
Liétor, J. (2014). Variabilidad intraespecífica de las conchas del género Iberus Montfort, 1810 del sur de la Península Ibérica. ConchBooks.
Liétor, J., Tudela, A. R., & Jódar, P. A. (2014). Variabilidad conquiológica de Iberus alcarazanus (Gastropoda, Stylommatophora, Helicidae) en el sur de Jaén. Zoologia Baetica, 25, 31–40.
López-Alcántara, A., Rivas, P., Alonso, M. R., & Ibáñez, M. (1985). Variabilidad de Iberus gualtieranus (Linneo, 1758) (Pulmonata, Helicidae). Iberus, 5, 83–112.
López-Villalta, J. S. (2011). Why mountain passes are higher for endemic snails. Zoologia Baetica, 22, 87–97.
Martínez-Ortí, F., & Robles, F. (2012). On the taxonomical identity of some taxa of the Iberian endemic genus Iberus Montfort, 1810 (Gastropoda, Helicidae). Animal Biodiversity and Conservation, 35, 99–105.
Miller, M. A., Pfeiffer, W., & Schwartz, T. (2010). Creating the CIPRES Science Gateway for inference of large phylogenetic trees. In 2010 Gateway computing environments workshop (GCE) (pp. 1–8). IEEE. https://doi.org/10.1109/GCE.2010.5676129
Montfort, P. (1810). Conchyliologie systématique et classification méthodique des coquilles (Vol. 2, p. 676 + 16). Schoell. http://www.biodiversitylibrary.org/bibliography/10571
Moreno-Rueda, G. (2011). Revisión del estatus de conservación del caracol amenazado Iberus gualtieranus gualtieranus. Zoologica Baetica, 22, 69–85.
Moreno-Rueda, G., & Pizarro, M. (2007). Census method for estimating the population size of the endemic and threatened land snail Iberus gualtieranus gualtieranus. Animal Biodiversity and Conservation, 30, 1–5.
Nantarat, N., Sutcharit, C., Tongkerd, P., Wade, C. M., Naggs, F., & Panha, S. (2019). Phylogenetics and species delimitations of the operculated land snail Cyclophorus volvulus (Gastropoda: Cyclophoridae) reveal cryptic diversity and new species in Thailand. Scientific Reports, 9, 7041.
García San Nicolás, E. (1957). Estudios sobre la biología, la anatomía y la sistemática del género Iberus Montfort, 1910. Boletín De La Real Sociedad Española De Historia Natural, 55, 199–390.
Pfenninger, M., Cordellier, M., & Streit, B. (2006). Comparing the efficacy of morphologic and DNA-based taxonomy in the freshwater gastropod genus Radix (Basommatophora, Pulmonata). BMC Evolutionary Biology, 6, 100.
Pfenninger, M., & Magnin, F. (2001). Phenotypic evolution and hidden speciation in Candidula unifasciata ssp. (Helicellinae, Gastropoda) inferred by 16S variation and quantitative shell traits. Molecular Ecology, 10, 2541–2554.
Quinn, G. P., & Keough, M. J. (2002). Experimental design and data analysis for biologists. Cambridge University Press.
Rambaut, A. (2018). FigTree-version 1.4.4, a graphical viewer of phylogenetic trees. http://tree.bio.ed.ac.uk/software/figtree/
Rafinesque, C. S. (1815). Analyse de la nature, ou tableau de l'univers et des corps organisés (p. 224). L'Imprimerie de Jean Barravecchia. https://www.biodiversitylibrary.org/page/48310197#page/11/mode/1up
Ronquist, F., & Huelsenbeck, J. P. (2003). MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572–1574.
Rossmässler, E. A. (1854). Iconographie der Land–und Süsswasser–Mollusken Europa’s, mit vorzüglicher Berücksichtigung kritischer und noch nicht abgebildeter Arten, 3(1–2): 1–31. Leipzig: H. Costenoble Ed.
Ruiz Ruiz, A., Cárcaba, A., Porras, A., & Arrébola, J. R. (2006). Caracoles terrestres de Andalucía. Guía y manual de identificación. Fundación Gypaetus.
Schmidt, F. J. (1855). Beschreibung zweier neuer Höhlenthiere, eines Käfers und einer Schnecke. Verhandlungen des Zoologisch-Botanischen Vereins in Wien, 5, 6. https://www.biodiversitylibrary.org/page/42798203. https://es.wikipedia.org/wiki/
Senar, J. C. (1999). La medición de la repetibilidad y el error de medida. Etologuía, 17, 53–64.
Silvestro, D., & Michalak, I. (2012). raxmlGUI: A graphical front-end for RAxML. Organisms Diversity and Evolution, 12, 335–337.
Teshima, H., Davison, A., Kuwahara, Y., Yokoyama, J., Chiba, S., Fukuda, T., Ogimura, H., & Kawata, M. (2003). The evolution of extreme shell shape variation in the land snail Ainohelix editha. Molecular Ecology, 12, 1869–1878.
Verdú, J. R., Numa, C., & Galante, E. (2011). Atlas y libro rojo de los invertebrados amenzados de España (especies vulnerables). Madrid: Dirección General de Medio Natural y Política Forestal, Ministerio de Medio Ambiente, Medio Rural y Marino.
Wilke, T., Pfenninger, M., & Davis, G. (2002). Statistical discrimination and evolutionary significance of anatomical variation in cryptic mudsnail species. Proceedings of the Academy of Natural Sciences of Philadelphia, 152, 45–66.
Zuur, A. F., Ieno, E. N., & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1, 3–14.
Acknowledgements
Our most sincere thanks to Arturo Sierra Lara, for the support provided with the review of the taxonomic history of I. alcarazanus. We are also immensely grateful to Mohammed Bakkali for his valuable support in the genetic analysis. This study has been carried out in accordance with both Spanish and Andalusian legislation (Law 8/2003) for the protection of wild fauna and flora in the case of invertebrate species not included in the National (Royal Decree 139/2011) and Andalusian (Decree 23/2012) catalogue of protected species. Comments by two anonymous referees contributed to improving the typescript.
Funding
Funding for open access publishing: Universidad de Granada/CBUA. MJJ was supported by a postdoctoral “María Zambrano” fellowship. Funding for open access charge: Universidad de Granada / CBUA.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the idea of the study. JL, ART and PAJ carried out the sampling. MJJ carried out the genetic analysis. All the authors discussed the results, defined the main conclusions of the study and contributed to the writing of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liétor, J., Tudela, A.R., Jódar, P.A. et al. Slow and steady saves the race: molecular and morphological analysis of three new cryptic species of Iberus land snails from the Iberian Peninsula. Org Divers Evol (2024). https://doi.org/10.1007/s13127-024-00640-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13127-024-00640-3