Abstract
Two new species of copepods assigned to the genus Acusicola Cressey, 1970 (Cyclopoida: Ergasilidae) are proposed based on post-metamorphic adult females, parasitizing the gills of two actinopterygian fish off Brazil namely, the Tripletail Lobotes surinamensis (Bloch) (Lobotidae), collected in the coastal zone of the State of Pará, near Curuçá Municipallity, and the Swordspine snook Centropomus ensiferus Poey (Centropomidae) collected in Sepetiba Bay, State of Rio de Janeiro, Brazil. Acusicola iamarinoi n. sp. parasite of L. surinamensis, differs from its closet congeners based on the first segment of the antennule armed with 10 setae, the presence of a maxillule armed with four elements and a pair of blunt processes dorsally on the fourth pedigerous somite. Acusicola pasternakae n. sp., collected from C. ensiferus, can be distinguished from its closest congeners based on the membranous sheath of the first endopodal segment of antenna with horizontal marks, the first segment of the antennule armed with 11 setae and a spine on the last exopodal segment of leg 2. This is the first report of representatives of Acusicola parasitizing fish of the families Lobotidae and Centropomidae as well as new geographical records of the genus in the coast of State of Pará and in Sepetiba Bay, Brazil.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Members of the family Ergasilidae Burmeister, 1835 represent one of the richest groups of crustacean parasites, currently including 265 species from 30 genera, distributed worldwide (Oliveira et al., 2021; Walter & Boxshall, 2022). Most of these copepods are found infesting the gills of freshwater actinopterygians, but some species may be attached to nostrils, tegument and urinary bladder and, less frequently, parasitizes brackish and marine fish, as well as mollusks (Boxshall & Halsey, 2004; Rosim et al., 2013; Taborda et al., 2016). Ergasilidae is the richest family of parasitic copepods found on fish from Brazil, on which 74 species from 18 genera have been reported (Narciso et al., 2019; Narciso & Da Silva, 2020; Narciso et al., 2020; Narciso et al. 2021a, 2021b; Oliveira et al., 2021). However, some authors affirm that such diversity is still underestimated, since only a small fraction of the Brazilian ichthyofauna has been studied for parasitic copepods (Luque et al., 2013; Taborda et al., 2016; Narciso & Da Silva, 2020; Paschoal et al., 2022).
The genus Acusicola Cressey, 1970 is currently known to comprise 15 species, eight of which have been reported in Brazil, especially in the Amazon basin (Amado & Rocha, 1996; Santacruz et al., 2020; Walter & Boxshall, 2022). These ergasilid copepods, together with Miracetyma Malta, 1993 and Amplexibranchius Thatcher & Paredes, 1985, belong to a group of genera that has peculiar attachment mechanisms, which is composed by a pair of antennae with short terminal claw that latches into a groove on the second endopodal segment of the opposite pair, enabling the completely involvement of the gill filament. However, species of Acusicola presents a 2-segmented endopod on the first pair of legs armed with at least six elements; a feature that is not found in the other two genera of the group (Malta, 1993; Thatcher & Paredes, 1985; Boxshall & Halsey, 2004). This genus was originally proposed to accommodate Acusicola tenax (Roberts, 1965), firstly assigned to Ergasilus von Nordmann, 1832, infesting gill filaments of Pomoxis annularis Rafinesque (Centrarchidae) in Garza-Little Elm Lake, Texas, USA (Roberts, 1965; Cressey & Collette, 1970). Subsequently, other species of Acusicola have been reported in the American continent, from the gills of different teleost families, especially Belonidae, Engraulidae, and Mugilidae, as well as from plankton samples (Santacruz et al., 2020). Recently, Santacruz et al. (2020) proposed a new species of Acusicola parasitizing the gills of Amphilophus citrinellus (Günther) (Cichlidae) in Nicaragua, using for the first time in Acusicola an integrative taxonomic approach.
During a parasitological survey of estuarine fish off Brazil, two species of parasitic copepods belonging to the genus Acusicola were found on the gills of two teleost hosts: Lobotes surinamensis (Bloch) (Lobotidae), from the coastal zone of the State of Pará, and Centropomus ensiferus Poey (Centropomidae) from Sepetiba Bay, State of Rio de Janeiro, Brazil. Detailed morphological study of these specimens revealed that they represent two unknown species, which are fully described herein.
Materials and Methods
Fish were bought from local fisherman in two different localities: eight specimens of L. surinamenis obtained in January 2021 in the coastal zone of the State of Pará (0°33′42″S, 47°51′00″W), Northern Brazil; and four specimens of C. ensiferus obtained between January 2020 to March 2020 in Sepetiba Bay (22°57′18″S, 43°54′44″W), State of Rio de Janeiro, Southeastern Brazil. Hosts were analyzed mostly fresh, but some specimens were kept frozen at -20°C, prior to parasitological examination. Copepods were collected from the gills, by careful washing of gill filaments in flowing water or delicate detaching using a thin needle, fixed and preserved in 70% ethanol. For microscopical observations, parasite specimens were cleared in 85% lactic acid and the appendages were dissected and examined using the wooden slide procedure described by Humes and Gooding (1964). Drawings were made with the aid of a Zeiss Standard 20 microscope (Carl Zeiss Foundation, Germany), equipped with a drawing tube. Measurements, all in micrometers unless otherwise stated, were made using an ocular micrometer and are given by range followed by the mean and standard deviation in parentheses. The descriptive terminology and classification of copepods follow Boxshall and Halsey (2004). Prevalence and intensity are used according to Bush et al. (1997). Host identification was based on the key by Menezes and Figueiredo (1980) and Figueiredo and Menezes (1980); their nomenclature and classification were updated according to FishBase (Froese and Pauly, 2022). Type specimens were deposited in the collection of the Museu de Zoologia da Universidade de São Paulo (acronym MZUSP), Brazil.
Systematics
Order Cyclopoida Burmeister, 1834
Family Ergasilidae Burmeister, 1835
Genus Acusicola
Cressey, 1970
Type-species: Acusicola cunula Cressey, 1970 by original designation.
Acusicola iamarinoi n. sp.
Type host: The Tripletail Lobotes surinamensis (Bloch) (Acanthuriformes: Lobotidae).
Prevalence: 100% (eight infested fish out of eight examined).
Mean intensity: mean of 11.1 copepods per infected fish (range 4–21).
Site of infection: Gills.
Type locality: Coastal zone of the State of Pará, near Curuçá Municipality, Brazil (0°33'42"S, 47°51'00"W).
Specimens deposited: Holotype female (MZUSP-43421) and 9 paratypes female (MZUSP-43422).
ZooBank registration: urn:lsid:zoobank.org:act:5F71F3A0-7693-4890-9EF1-8872A4BB13C1.
Etymology: The new species is named in honor of Dr. Átila Iamarino from Brazil, for his contribution to science communication, especially during the COVID-19 pandemic.
Description
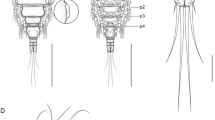
Adult female [based on 10 specimens; Figs. 1–3]. Body length from anterior margin of prosome to posterior margin of caudal rami 560–720 (654 ± 51.6). Body comprising prosome and urosome (Figs. 1A, B); prosome consisting of cephalosome, with antennule visible only in dorsal view and 4 pedigerous somites. Cephalosome and first pedigerous somite not fused (Figs. 1A, B). Cephalosome (Fig. 1A) longer than wide, 200–250 (220 ± 17.3) × 140–210 (181 ± 22.7), not inflated and slightly constricted, representing more than one third of body length; dorsal surface of cephalosome with inverted T-shaped mark (Fig. 1A). Depression between cephalosome and first pedigerous somite, with posterior margin of cephalosome distinct in both lateral and dorsal views (Figs. 1A, B). Fourth pedigerous somite ornate with pair of blunt processes on dorsal part of anterior margin (Figs. 1A, B). Urosome consisting of fifth pedigerous somite, genital double-somite, and 3 free abdominal somites; third abdominal somite (= anal somite) bipartite. Fifth pedigerous somite (Fig. 1C) short, with row of spinules on medio-ventral surface. Genital double-somite (Fig. 1C) longer than wide, 60–80 (69 ± 6.9) × 55–75 (66 ± 4.8), with ventral surface ornate with 2 plates of spinules on medio-ventral surface and row of spinules along posteroventral margin. Free abdominal somites (Fig. 1C) wider than long; first and second nearly equal in length; third somite smaller than previous two. Posteroventral and posterolateral margins of abdominal somites ornamented by row of spinules each; third somite with additional row of spinules on inner lateral margin.
Acusicola iamarinoi n. sp. (adult female). A, habitus, dorsal, ts = T-shaped mark; B, habitus, lateral, a1 = antennule, a2 = antenna, p1 = leg 1, p2 = leg 2, p3 = leg 3, p4 = leg 4, p5 = leg 5, pp = prosomal process; C, fifth pedigerous somite, abdomen and caudal rami, ventral; D, antennule, ventral, ae = aesthetascs. Scale bars: A–B = 200 µm; C = 50 µm; D = 25 µm
Caudal rami (Fig. 1C) longer than third abdominal somite; with row of spinules on ventral surface extending near posterior and medial margins; each ramus armed with 2 large medial setae, 1 minor medial seta and 1 seta at outer corner. Two egg-sacs (Fig. 2D), much longer than wide, each composed by 2–4 rows of eggs.
Acusicola iamarinoi n. sp. (adult female). A, antenna, ventral, fo = fossae, th = vestigial third endopodal segment, gr = groove; B, mouthparts, ventral, cp = chitinous process, mb = mandible, me = maxillule, sy = syncoxa, pr = protrusion; C, interpodal plates of legs 1 to 4, ventral; D, egg sac, dorsal; E, leg 5, lateral. Scale bars: A = 100 µm; B = 50 µm; C = 30 µm; D = 200 µm; E = 20 µm
Antennule 5-segmented (Fig. 1D), tapering distally, aesthetascs present on fourth and fifth segments; setal formula as follows: 10: 4: 4: 2 + ae: 5 + ae: all setae naked. Antenna (Fig. 2A) comprising coxobasis and 3-segmented endopod with terminal claw; coxobasis, first endopodal segment and the first half of second endopodal segment enclosed by membranous sheath. Coxobasis short, proximally longer, armed with modified peg seta on inner distal surface; membrane between coxobasis and first endopodal segment not inflated. First endopodal segment longest, nearly 2.4× longer than coxobasis, armed with 2 spiniform elements, 1 proximal on outer margin and 1 distal on medial margin, with hyaline processes along inner margin;outer margin of membranous sheath ornamented by small setules; second endopodal segment longer than wide, about 1.4× shorter than coxobasis, groove on the anterior margin, near half of segment; third endopodal segment vestigial, bearing short, curved claw with fossae on inner margin, near tip.
Mouthparts (Fig. 2B) include mandible, maxillule and maxilla; maxilliped absent. Mandible unsegmented, with anterior chitinous process, bearing palp and mid and posterior blades, anterior blade not observed; palp small and naked; mid blade with long spines in outer margin; posterior blade with smooth teeth along posterior margin. Maxillule small, bearing 3 unequal outer setae and 1 inner spiniform element. Maxilla comprising large syncoxa with 1 seta near basis and protrusion in the posterior margin near teeth; second segment (basis) bearing long and sharp anterior teeth with long spinules along anterior and apical margins.
Swimming legs 1–4 biramous (Figs. 3A–D), each with 2-segmented protopod comprising coxa and basis; interpodal plates (Fig. 2C) with row of spinules (legs 2 and 3) or smooth (legs 1 and 4). Armature of legs (spines, Roman numerals; setae, Arabic numerals) as follows:
Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
Leg 1 | 0-0 | 0-1 | I-0; 0-1; II-5 | 0-1; II-4 |
Leg 2 | 0-0 | 0-1 | I-0; 0-1; I-6 | 0-1; 0-2; I-4 |
Leg 3 | 0-0 | 0-1 | I-0; 0-1; 0-6 | 0-1; 0-2; I-4 |
Leg 4 | 0-0 | 0-1 | 0-0; 0-5 | 0-1; 0-2; I-3 |
Leg 1 (Fig. 3A) coxa unarmed. Basis with outer naked seta. Exopod 3-segmented, with rows of spinules on outer margin of all segments; first segment with small outer spine; second segment with inner plumose seta; third segment with small subapical spine, long apical spine and 5 plumose setae. Endopod 2-segmented, both segments with rows of spinules on outer margin; first segment about 1.3× longer than exopodal ramus, with plumose inner seta; second segment with 4 plumose setae, 1 falciform subapical spine with hook near basis and 1 pectinate apical spine.
Leg 2 (Fig. 3B) coxa unarmed, with small protrusion on posterior margin. Basis with outer naked seta. Exopod 3-segmented, lacking spinules; first segment longest, with small outer spine; second segment with inner plumose seta; third segment shortest, with 6 apical plumose setae and 1 small outer spine. Endopod 3-segmented, with row of spinules on outer margin of second and third segments; first segment longest, with plumose inner seta; second segment with 2 plumose inner setae; third segment with subapical curved spine, and 4 plumose setae.
Leg 3 (Fig. 3C) similar to Leg 2, except for absence of outer spine on last exopodal segment and spinules on anterior outer margin of second endopodal segment.
Leg 4 (Fig. 3D) coxa unarmed with small protrusion on posterior margin. Basis with outer naked seta. Exopod 2-segmented, lacking spinules; first segment longest, unarmed; second segment with 5 long plumose apical setae. Endopod 3-segmented; first segment with patch of spinules on posteroventral and outer margins and 1 inner plumose seta; second segment with small patch of spinules on medioventral margin and 2 inner plumose setae; third segment with row of spinules on anterior ventral margin, 3 plumose setae and 1 long spine, about 1.4× shorter than endopodal ramus.
Leg 5 (Fig. 2E) represented by 2 naked setae, ventral seta longest; each carried on separate papilla.
Remarks
The new species differs from all congeners by its possession of a maxillule armed with four elements (i.e., three regular setae and one spiniform element), since the maxillule of the other known species has two (as in A. cunula Cressey, 1970, A. lycengraulidis Thatcher & Boeger, 1983a, 1983b, A. paracunula Amado & Rocha, 1996, A. pellonidis Thatcher & Boeger, 1983a, 1983b, A. rogeri Amado & Rocha, 1996, A. rotunda Amado & Rocha C.E.F., 1996, A. spinulosa Amado & Rocha C.E.F., 1996, A. tenax (Roberts, 1965) and A. tucunarense Thatcher, 1984) or three elements (as in A. braziliensis Amado & Rocha C.E.F., 1996, A. joturicola El-Rashidy & Boxshall, 1999, A. margulisae Santacruz, Morales-Serna, Leal-Cardín, Barluenga & de León, 2020, A. mazatlanensis El-Rashidy & Boxshall, 1999, A. minuta Araújo & Boxshall, 2001, A. spinuloderma El-Rashidy & Boxshall, 1999) (Cressey & Collette, 1970; Thatcher & Boeger, 1983a; Thatcher & Boeger, 1983b; Thatcher, 1984; Amado & Rocha, 1996; El-Rashidy & Boxshall, 1999; Araújo & Boxshall, 2001; Santacruz et al. 2020). Acusicola iamarinoi n. sp. has the following feature that may be considered an autapomorphy among species of Acusicola: a pair of dorsal blunt processes on the anterior margin of the fourth pedigerous somite. Therefore, the new species is easily diagnosed based on this autapomorphy and, consequently, clearly differs from the congeners.
Acusicola margulisae, a parasite of A. citrinellus from Nicaragua, shares with A. iamarinoi n. sp. a long first endopodal segment in leg 1, about 1.3× longer than the exopodal ramus. The new species differs from A. margulisae because its cephalosome is clearly separated from the first pedigerous somite (vs fused in the latter), its first antennulary segment is armed with 10 setae (vs 12 seta in the latter), the second endopodal segments of legs 1 and 2 lacking processes (vs present in the latter) and by the caudal rami with two long setae (vs one seta in the latter) (Santacruz et al., 2020).
Acusicola iamarinoi n. sp. resembles A. brasiliensis, A. minuta A. spinuloderma and A. tenax by sharing the presence of a long spine on the terminal segment of the endopod of leg 4. However, the new species differs from these congeners because it has the first antennulary segment armed with 10 setae (vs 11 in A. brasiliensis, 12 in A. minuta and A. spinuloderma, and 13 in A. tenax). Moreover, it differs from A. brasiliensis and A. spinuloderma in the absence of a spine on the last exopodal segment of leg 3 (vs presence in the latter two). Acusicola iamarinoi n. sp. also differs from A. minuta by the antenna lacking two membranous expansions on the inner margin of the second endopodal segment (vs presence in the latter); from A. spinuloderma by having the cephalosome clearly separated from the first pedigerous somite (vs fused in the latter); and from A. tenax by the presence of a spine on the first exopodal segment of leg 1 (vs absence in the latter) (Roberts, 1965; Amado & Rocha, 1996; El-Rashidy & Boxshall, 1999; Araújo & Boxshall, 2001).
Acusicola pasternakae. n. sp
Type host: The Swordspine snook Centropomus ensiferus Poey (Carangaria: Centropomidae).
Prevalence: 50% (two infested fish out of four examined).
Mean intensity: mean of 4 copepods per infected fish (range 2–6).
Site of infection: Gills.
Type locality: Sepetiba Bay, State of Rio de Janeiro, Brazil (22°57'18"S, 43°54'44"W).
Specimens deposited: Holotype female (MZUSP-43423) and 4 paratypes female (MZUSP-43424).
ZooBank registration: urn:lsid:zoobank.org:act:81615CB6-A754-457E-BBF0-33B54052AA1D
Etymology: The new species is named in honor of Dr. Natalia Pasternak Taschner from Brazil, for her contribution to science communication, especially during the COVID-19 pandemic.
Description
Adult female [based on 6 specimens; Figs. 4–6]. Body length from anterior margin of prosome to posterior margin of caudal rami 800–1000 (908 ± 82.6). Body comprising prosome and urosome (Figs. 4A, B); prosome consisting of cephalosome, with antennule visible only in dorsal view and 4 pedigerous somites. Cephalosome and first pedigerous somite not fused (Figs. 4A, B). Cephalosome (Fig. 4A) longer than wide, 250–340 (307 ± 31.4) × 200–255 (226 ± 22), not inflated and slightly constricted, representing more than one third of body length; dorsal surface of cephalosome with nauplius eye near anterior margin, inverted T-shaped mark and pair of sensilla (Figs. 4A). Depression between cephalosome and first pedigerous somite, with posterior margin of cephalosome distinct in both lateral and dorsal views (Figs. 4A, B). Second and third pedigerous somites ornate with 1 medial sensillum each (Figs. 4A, B). Urosome consisting of fifth pedigerous somite, genital double-somite, and 3 free abdominal somites; third abdominal somite (= anal somite) bipartite. Fifth pedigerous somite (Fig. 4C) short. Genital double-somite (Fig. 4C) longer than wide, 90–100 (97.2 ± 4.5) × 84–95 (89 ± 4), with row of spinules on medio-ventral surface and row of spinules along posteroventral margin. Free abdominal somites (Fig. 4C) wider than long; first somite longer than second; third somite smaller than previous two. First abdominal somite with row of spinules on posteroventral margin; second abdominal somite with 2 patches of spinules on anterior and posterolateral margins of ventral surface and 1 row of spinules on posteroventral margin; third somite with patch of spinules on medio-lateral margin.
Acusicola pasternakae n. sp. (adult female). A, habitus, dorsal, ts= T-shaped mark; B, habitus, lateral, ne = nauplius eye, a1 = antennule, a2 = antenna, se = sensillum, p1 = prosome 1, p2 = leg 2, p3 = leg 3, p4 = leg 4, p5 = leg 5; C, fifth pedigerous somite, abdomen and caudal rami, ventral; D, antennule, ventral, ae = aesthetascs. Scale bars: A–B = 300 µm; C = 100 µm; D = 50 µm
Caudal rami (Fig. 4C) longer than third abdominal somite; each ramus armed with 1 large medial seta, 2 unequal ventral setae and 1 seta at outer corner. Two egg-sacs (Fig. 5D), much longer than wide, each composed by 2–4 rows of eggs.
Acusicola pasternakae n. sp. (adult female). A, antenna, ventral, fo = fossae, th = vestigial third endopodal segment, gr = groove; B, mouthparts, ventral, mb = mandible, me = maxillule, sy = syncoxa; C, interpodal plates of legs 1 to 4, ventral; D, egg sac, dorsal; E, leg 5, lateral. Scale bars: A = 200 µm; B = 50 µm; C = 30 µm; D = 400 µm; E = 15 µm
Antennule 5-segmented (Fig. 4D), tapering distally, aesthetascs present on fourth and fifth segments; setal formula as follows: 11: 3: 4: 2 + ae: 6 + ae: all setae naked. Antenna (Fig. 5A) comprising coxobasis and 3-segmented endopod with terminal claw; coxobasis, first endopodal segment and the first half of the second endopodal segment enclosed by membranous sheath. Coxobasis short, proximally longer, armed with modified peg seta on inner distal surface; membrane between coxobasis and first endopodal segment not inflated. First endopodal segment longest, nearly 2.6× longer than coxobasis, armed with anterior spiniform element and posterior blunt element with thick spine at base, with hyaline processes along inner margin; membranous inner margin of membranous sheath ornamented with horizontal marks. Second endopodal segment longer than wide, about 1.2× longer than coxobasis, groove on the anterior margin, near half of segment. Third endopodal segment vestigial, bearing short, curved claw with fossae on inner margin near tip.
Mouthparts (Fig. 5B) include mandible, maxillule and maxilla; maxilliped absent. Mandible unsegmented, bearing palp and mid and posterior blades; palp small and naked, anterior blade not observed; mid blade with long spines in posterior margin; posterior blade with smooth teeth along posterior margin. Maxillule small, bearing 2 setae similar in size. Maxilla comprising large syncoxa with 2 setae, 1 on posterior margin and 1 near basis; second segment (basis), bearing long, sharp anterior teeth with long spinules along anterior and apical margins.
Swimming legs 1–4 biramous (Figs. 6A–D), each with 2-segmented protopod comprising coxa and basis; interpodal plates (Fig. 5C) with row of spinules (legs 1, 2 and 3) or smooth (leg 4). Armature of legs (spines, Roman numerals; setae, Arabic numerals) as follows:
Coxa | Basis | Exopod | Endopod | |
|---|---|---|---|---|
Leg 1 | 0-0 | 0-1 | I-0; 0-1; II-5 | 0-1; II-4 |
Leg 2 | 0-0 | 0-1 | I-0; 0-1; I-6 | 0-1; 0-2; I-4 |
Leg 3 | 0-0 | 0-1 | I-0; 0-1; 0-6 | 0-1; 0-2; I-4 |
Leg 4 | 0-0 | 0-1 | 0-0; 0-4 | 0-1; 0-2; I-3 |
Leg 1 (Fig. 6A) coxa unarmed with small protrusion on posterior margin. Basis with outer naked seta. Exopod 3-segmented, with rows of spinules on outer margin of all segments, reaching ventral surface on last segment; first segment with small outer spine; second segment with inner plumose seta; third segment with 2 apical spines of equal size and 5 plumose setae. Endopod 2-segmented, both segments with rows of spinules on outer margin; first segment about 1.3× shorter as exopodal ramus, with plumose inner seta; second segment with 4 plumose setae, 1 falciform subapical spine with a hook near basis and 1 pectinate apical spine, both with spinules on outer margin.
Leg 2 (Fig. 6B) coxa unarmed with small protrusion on posterior margin. Basis with outer naked seta. Exopod 3-segmented, lacking spinules; first segment longest, with small outer spine; second segment with inner plumose seta; third segment shortest, with 6 apical plumose setae and 1 small outer spine. Endopod 3-segmented, lacking spinules; first segment longest, with plumose inner seta; second segment with 2 plumose inner setae; third segment with subapical spine and 4 plumose setae.
Leg 3 (Fig. 6C) similar to Leg 2, except for absence of outer spine on last exopodal segment.
Leg 4 (Fig. 6D) coxa unarmed. Basis with outer naked seta and patch of spinules on posterior margin, near endopod attachment site. Exopod 2-segmented, lacking spinules; first segment longest, unarmed; second segment with 4 long, plumose apical setae. Endopod 3-segmented; first segment lacking spinules, with 1 inner plumose seta; second segment with row of spinules on posteroventral margin and 2 inner plumose setae; third segment with row of spinules on outer margin, patch of spinules on posteroventral surface, armed with 3 lateral plumose setae and 1 long apical spine about 2.1× shorter than endopodal ramus.
Leg 5 (Fig. 5E) represented by 2 naked setae, ventral seta longest; each inserted on separate papilla.
Remarks
Of the 15 nominal species assigned to Acusicola, only three have the last exopodal segment of leg 4 armed with four setae as in A. pasternakae n. sp. namely, A. rogeri, A. spinulosa and A. tenax. However, the new species differs from these closely species because in its antennule the first segment is armed with 11 setae (vs eight setae in A. rogeri and A. spinulosa and 13 setae in A. tenax) and its membranous sheath of the first endopodal segment of antennae has horizontal markings (vs absence of it in the last three species) (Roberts, 1965; Cressey & Collette, 1970; Amado & Rocha, 1996; Santacruz et al., 2020). The new species also differs from A. rogeri and A. spínulosa by the presence of a spine on the last exopodal segment of legs 2 and 3 (vs absence in the latter) (Cressey & Collette, 1970; Amado & Rocha, 1996).
In addition, Acusicola pasternakae n. sp. differs from A. tenax by having a spine on the first exopodal segment of leg 1 (vs absence in the latter). Moreover, A. pasternakae n. sp. differs from A. spinulosa because its cephalossome is separated from the first pedigerous somite (vs not separated in the latter), last endopodal segment of leg 1 with four setae and two spines (vs three reduced spines in the latter), last endopodal segment of leg 4 with three setae (vs four setae in the latter) and leg 5 reduced to two setae (vs leg 5 reduced to a single seta in the latter) (Roberts, 1965; Amado & Rocha, 1996).
Discussion
The two new species proposed in the present study were assigned to Acusicola based on the following features present in the parasitic females: a 5-segmented antennule, antennae with short curved apical claw that latches into a groove on the second endopodal segment of the opposite antenna, and a 2-segmented endopod with at least six elements on leg 1 (Cressey & Collette, 1970, Boxshall & Halsey, 2004). According to Santacruz et al. (2020), species of Acusicola parasitize a wide range of actinopterygian hosts, especially members of the families Belonidae, Engraulidae and Mugilidae, but some species are present in fish of the families Atherinopsidae, Cichlidae, Clupeidae, Poeciliidae, Pristigasteridae, and Centrarchidae. Currently, only one representative of Ergasilidae has been reported infesting species belonging to Lobotidae, i.e., Ergasilus monodi Brian, 1927 on L. surinamensis from Cameroon. In contrast, there are three reports of ergasilids infesting Centropomidae fish: Ergasilus sp. on Centropomus undecimalis (Bloch) from Brazil, Ergasilus davidi Suárez-Morales & Santana-Piñeros, 2008 and Therodamas mexicanus Suárez-Morales, Santana-Piñeros & González-Solís, 2008 on Centropomus robalito Jordan & Gilbert from Mexico (Brian, 1927; Tavares & Luque, 2004; González-Solís et al., 2008; Suárez-Morales & Santana-Piñeros, 2008). Consequently, the present results, represent the first report of the genus Acusicola infesting fish of the families Lobotidae and Centropomidae in the world, suggesting that these two families include potential hosts for ergasilid copepods.
Tavares and Luque (2004) examined 79 specimens of C. undecimalis from the same locality as the one of the present study (Sepetiba Bay, State of Rio de Janeiro), reporting Ergasilus sp. on the gills. In order to confirm the identity of these specimens, the vouchers deposited in the Coleção Carcinológica do Museu Nacional do Rio de Janeiro (accession number MNRJ-15426) were requested for analysis. Unfortunately, the curator of MNRJ informed that the material was lost during a fire that committed the museum in September 2018. Since C. undecimalis and C. ensiferus are sympatric in Sepetiba Bay, it is possible that the Ergasilus sp. reported by Tavares and Luque (2004) is conspecific to A. pasternakae n. sp.; however, in order to confirm such hypothesis, new collections of ergasilid copepods from the gills of C. undecimalis in Sepetiba Bay are necessary.
Since the erection of Acusicola species have been mostly reported from freshwater environment, but some infest catadromous hosts (Santacruz et al., 2020). Acusicola brasiliensis, for example, has been reported infesting Lile piquitinga (Schreiner & Miranda Ribeiro), a clupeid fish that occurs inshore, on muddy bottoms, as well as in brackish or moderately saline coastal lagoons. This ergasilid can be found on different types of habitats similar to its host, being reported from freshwater habitats in Alegre, State of Pará, brackish and marine conditions in Cambori Beach, State of Espírito Santo, Itaparica Island, State of Bahia, and São Cristovão Beach, State of Sergipe, all in Brazil (Amado & Rocha, 1996; Froese & Pauly, 2022). The Tripletail L. surinamensis has similar biology as L. piquitinga, in which adults inhabit bays, muddy estuaries and lower reaches of large rivers. Similarly, the Swordspine snook C. ensiferus, can be found in coastal waters, estuaries and lagoons, migrating to freshwater, usually preferring habitats with low salinity (Froese & Pauly, 2022). Although the two new species of copepods described in the present study were originally found from brackish waters, their habitat might be similar to that of the hosts, i.e., extending from freshwater to brackish and even more saline habitats.
Ergasilids represent the second most reported group of parasitic copepods infesting marine and brackish farmed fish, frequently causing tissue damage and economic losses (Thatcher, 1998; Johnson et al., 2004; Pádua et al., 2015). Within the Neotropical Region, Brazil has the greatest species richness of parasitic crustaceans, most of which are ergasilid copepods (Luque et al., 2013). Despite the frequent occurrence and impact in aquaculture, the knowledge pertaining to the diversity and distribution of these parasites is still underestimated, since less than 10% of the local ichthyofauna has been studied for parasitic copepods (Luque et al., 2013; Couto & Paschoal, 2021; Paschoal et al., 2022; Narciso et al., 2022). Such gapped knowledge is concerning, since aquiculture has been expressively expanding during the last years (see FAO, 2021), which also highlights the need for further investigations on Ergasilidade, as well as on other groups of parasitic copepods from fish in Brazil.
References
Amado, M. A. P. M., & Rocha, C. E. F. (1996). New species of parasitic copepods of the Genus Acusicola (Poecilostomatoida: Ergasilidae) from gill filaments of coastal and freshwater Brazilian fishes, and proposition of Acusicola rogeri n. sp. for A. tenax sensu Cressey & Collette (1970). Hydrobiologia¸ 324(3), 183–193. https://doi.org/10.1007/bf00016390
Araújo, H. M. P., & Boxshall, G. A. (2001). A new species of Acusicola Cressey (Copepoda: Ergasilidae) from northeastern Brazil. Systematic Parasitology, 49(2), 149–157. doi: https://doi.org/10.1023/a:1010624822047
Boxshall, G. A., & Halsey, S. H. (2004). An introduction to copepod diversity. The Ray Society.
Brian, A. (1927). Crustacea II. Copepoda parasitica. In: Monod, T. (Ed.), Contribution a I'etude de la faune du Cameroun (1th ed., pp. 570-587). Éditeur Scientifique.
Bush, J. O., Lafferty, K. D., Lotz, J. M., & Shostak, A. W. (1997). Parasitology meets ecology on its own terms: Margolis et al. revisited. Journal of Parasitology, 83(4), 575–583. doi: https://doi.org/10.2307/2F3284227
Cressey, R., & Collette, B. (1970). Copepods and needlefishes: A study in host-parasite relationships. Fishery Bulletin, 68, 347–432.
Couto, J. V., & Paschoal, F. (2021). Two new species of Colobomatus Hesse, 1873 (Crustacea: Philichthyidae) parasitic in the interorbital canals of Holocentrus spp. (Holocentriformes: Holocentridae) in the South Atlantic Ocean. Systematic Parasitology, 98(5–6), 753–764. https://doi.org/10.1007/s11230-021-10009-1
El-Rashidy, R. H., & Boxshall, G. A. (1999). Ergasilid copepods (Poecilostomatoida) from the gills of primitive Mugilidae (grey mullets). Systematic Parasitology, 42, 161–186. doi: https://doi.org/10.1023/a:1006075223683
Food and Agriculture Organization of the United Nations. (2021). Yearbook of Fishery and Aquaculture Statistics.
Figueiredo, J. L., & Menezes, N. A. (1980). Manual de Peixes Marinhos do Sudeste do Brasil. III. Teleostei (2). Museu de Zoologia da Universidade de São Paulo.
Froese, R., & Pauly, D. (2022). FishBase. World Wide Web electronic publication. http://www.fishbase.org, version 02/2022. Accessed on 2022-09-20.
González-Solís, D., Suárez-Morales, E., & Santana-Piñeros, A. (2008). A new species and host range of Therodamas (Copepoda, Ergasilidae) from the Eastern Tropical Pacific, Crustaceana, 81(9), 1107–1117. doi: https://doi.org/10.1163/156854008X360842
Humes, A., & Gooding, R. (1964). A method for studying the external anatomy of copepods. Crustaceana, 6(3), 238–240. doi: https://doi.org/10.1163/156854064x00650
Johnson, S. C., Treasurer, J. W., Bravo, S., Nagasawa, K., & Kabata, Z. (2004). A Review of the Impact of Parasitic Copepods on Marine Aquaculture. Zoological Studies, 43(2), 229-243.
Luque, J. L., Vieira, F. M., Takemoto, R. M., Pavanelli, G. C., & Eiras, J. C. (2013). Checklist of Crustacea parasitizing fishes from Brazil. Check List, 9(6), 1449–1470. doi: https://doi.org/10.15560/9.6.1449
Malta, J. C. O. (1993). Miracetyma etimaruya gen. et sp. n. (Copepoda, Poecilostomatoida, Ergasilidae) From Freshwater Fishes Of The Brazilian Amazon. Acta Amazonica, 23(1), 49–57. doi: https://doi.org/10.1590/1809-43921993231057
Menezes, N. A., & Figueiredo, J. L. (1980). Manual de Peixes Marinhos do Sudeste do Brasil. IV. Teleostei (3). Museu de Zoologia da Universidade de São Paulo.
Narciso, R. B., Brandão, H., & Perbiche-Neves, G. (2019). A New Genus of Ergasilidae (Copepoda: Cyclopoida) from the Gills of Astyanax fasciatus (Cuvier, 1819) (Actinopterygii: Characidae). Acta Parasitologica, 64(4), 850–865. doi: https://doi.org/10.2478/s11686-019-00108-x
Narciso, R. B., & Da Silva, R. J. (2020). Two Gamispatulus Thatcher & Boger, 1984 (Cyclopoida: Ergasilidae) from Schizodon intermedius Garavello & Britski (Actinopterygii: Anostomidae), with description of a new species. Zootaxa, 4803(3), 463–482. doi: https://doi.org/10.11646/zootaxa.4803.3.3
Narciso, R. B., Perbiche-Neves, G., & Silva, R. J. (2020). A new species of Pseudovaigamus Amado, Ho & Rocha, 1984 (Cyclopoida: Ergasilidae) from the teleost Pimelodus maculatus Lacepède, 1803. Zootaxa, 4881(2), 349–360. doi: https://doi.org/10.11646/zootaxa.4881.2.8
Narciso, R. B., Brandão, H., Perbiche-Neves, G., & Silva, R. J. (2021a.). A New Species of Rhinergasilus Boeger et Thatcher, 1988 (Copepoda: Ergasilidae) from Gills of Astyanax fasciatus (Cuvier, 1819) (Actinopterygii: Characidae). Acta Parasitologica, 65(2), 327–334. doi: https://doi.org/10.2478/s11686-020-00168-4
Narciso, R. B., Perbiche-Neves, G., & Da Silva, R. J. (2021b). Rhinergasilus unguilongus n. sp. (Copepoda: Ergasilidae): A Gill Parasite of the Freshwater Fish Prochilodus lineatus (Valenciennes, 1837) (Actinopterygii: Prochilodontidae) from the Neotropical Region, Brazil. Acta Parasitologica, 66(1), 155–162. doi: https://doi.org/10.1007/s11686-020-00270-7
Narciso, R. B., Vieira, D. H. M. D., & da Silva, R. J. (2022). A new species of Tiddergasilus Marques & Boeger, 2018 (Copepoda: Ergasilidae) from the gills of Astyanax lacustris (Lütken) (Osteichthyes: Characidae) in Brazil. Systematic Parasitology (available online). doi: https://doi.org/10.1007/s11230-022-10055-3
Oliveira, M. S. B., Corrêa, L. L., Adriano, E. A., & Tavares-Dias, M. (2021). Integrative taxonomy of a new species of Therodamas (Ergasilidae) infecting the Amazonian freshwater fish Leporinus fasciatus (Anostomidae). Parasitology Research, 120(9), 3137–3147. doi: https://doi.org/10.1007/s00436-021-07256-y
Pádua, S. B., Jerônimo, G. T., Menezes-Filho, R. N., Taboga, S. R., Martins, M. L., & Belo, M. A. A. (2015). Pathological assessment of farmed yellowtail tetra Astyanax altiparanae infested by Acusicola sp. (Ergasilidae). Aquaculture Reports, 2, 63–66. doi: https://doi.org/10.1016/j.aqrep.2015.08.003
Paschoal, F., Couto, J. V., Pereira, F. B., & Luque, J. L. (2022). A New Species of Hatschekiid Copepod (Crustacea: Hatschekiidae) Parasitic on the Porkfish Anisotremus virginicus (Linnaeus, 1758) (Actinopterygii: Haemulidae), with Notes on Previously Known Species of Hatschekia Poche, 1902 Collected from Actinopterygians off Brazil. Acta Parasitologica, 67(3), 1126–1135. doi: https://doi.org/10.1007/s11686-022-00551-3
Roberts, L. S. (1965). Ergasilus tenax sp. n. (Copepoda: Cyclopoida) from the White Crappie, Pomoxis annularis Rafinesque. The Journal of Parasitology, 51(6), 987–989. doi: https://doi.org/10.2307/3275889
Rosim, D. F., Boxshall, G. A., & Ceccarelli, P. S. (2013). A novel micro-habitat for parasitic copepods: a new genus of Ergasilidae (Copepoda: Cyclopoida) from the urinary bladder of a freshwater fish. Parasitology International, 62(4), 347–354. doi: https://doi.org/10.1016/j.parint.2013.03.003
Santacruz, A., Morales-Serna, F. N., Leal-Cardín, M., Barluenga, M., & Léon, G. P. (2020). Acusicola margulisae n. sp. (Copepoda: Ergasilidae) from freshwater fishes in a Nicaraguan crater lake based on morphological and molecular evidence. Systematic Parasitology, 97(2), 165–177. doi: https://doi.org/10.1007/s11230-020-09906-8
Suárez-Morales, E., & Santana-Piñeros, A. M. (2008). A new species of Ergasilus (Copepoda: Cyclopoida: Ergasilidae) from coastal fishes of the Mexican Pacific. Folia Parasitologica 55(3), 224-230. doi: https://doi.org/10.14411/fp.2008.030
Taborda, N., Paschoal, F., & Luque, J. L. (2016). A new species of Ergasilus (Copepoda: Ergasilidae) from Geophagus altifrons and G. argyrostictus (Perciformes: Cichlidae) in the Brazilian Amazon. Acta Parasitologica, 61(3), 549–555. doi: https://doi.org/10.1515/ap-2016-0073
Tavares, L. E. R., & Luque, J. L. (2004). Community ecology of metazoan parasites of the later juvenile common snook Centropomus undecimalis (Osteichthyes: Centropomidae) from the coastal zone of the state of Rio de Janeiro, Brazil. Brazilian Journal of Biology, 64(3), 523-529. doi: https://doi.org/10.1590/S1519-69842004000300015
Thatcher, V. (1998). Copepods and fishes in the Brazilian Amazon. Journal of Marine Systems, 15(1-4), 97–112. doi: https://doi.org/10.1016/s0924-7963(97)00043-2
Thatcher, V., & Boeger, W. (1983a). The Parasitic Crustaceans of Fishes from the Brazilian Amazon, 8, Acusicola lycengraulidis n. sp. (Copepoda: Cyclopidea) from Lycengraulis grossidens Cuvier. Acta Amazonica, 13(5–6), 943–951. doi: https://doi.org/10.1590/1809-439219831356943
Thatcher, V., & Boeger, W. (1983b). The Parasitic Crustaceans of Fishes from the Brazilian Amazon, 10, Acusicola pellonidis n. sp. (Copepoda: Cyclopidea) from Pellona castelnaeana (Valenciennes). Amazoniana, 8(2), 273–279.
Thatcher, V., Paredes, V. (1985). A Parasitic Copepod, Amplexibranchius bryconis gen. et sp. nov. (Ergasilidae: Acusicolinae), from an Amazonian Fish and Remarks on the Importance of Leg Morphology in this Subfamily. Amazoniana, 9(2), 205–214.
Walter, T. C., & Boxshall, G. (2022). World of Copepods Database. Ergasilidae Burmeister, 1835. WoRMS. https://www.marinespecies.org/copepoda/aphia.php?p=taxdetails&id=128571. Accessed on 2022-09-20
Acknowledgments
Authors would like to thank the students of biology Nagylla de Fátima da Silva Sena and Julie Costa Rodrigues from Instituto Federal do Pará, as well as Larissa Maia from the Laboratory of Paleoparasitology, Instituto Oswaldo Cruz (acronym FIOCRUZ), Brazil, for collecting the parasite samples.
Funding
JVC was supported by Coordenação de Aperfeiçoamento de Pessoal do Ensino Superior (CAPES), Brazil. JLL was supported by Conselho Nacional de Desenvolvimento Científico e Tecnologico do Brasil (CNPq), Brazil. FP was supported by Fundação de Amparo à Pesquisa e ao Desenvolvimento Científico e Tecnológico do Maranhão (FAPEMA, process no. 84516/2022), Brazil.
Author information
Authors and Affiliations
Contributions
ANP, JLL and FP performed field collection and parasitological survey. JVC, FP and FBP analyzed the copepods, prepared the illustrations and wrote the first draft of the manuscript. All reviewed the manuscript and approved the final version. FBP and FP supervised the study.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable institutional, national and international guidelines for the care and use of animals were followed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Couto, J.V., de Nazaré Pereira, A., Luque, J.L. et al. Two new species of Acusicola Cressey, 1970 (Copepoda:Cyclopoida: Ergasilidae) parasitic on the gills of two estuarine actinopterygians off Brazil. Syst Parasitol 100, 133–148 (2023). https://doi.org/10.1007/s11230-022-10076-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-022-10076-y