Abstract
Echinocephalus caniculus n. sp. (Nematoda, Gnathostomatidae Railliet, 1895) was isolated from the spiral valve of the lesser spotted dogfish Scyliorhinus canicula (L.) from the waters off Kalaat El Andalous, North East Tunisia. This new species is mainly characterized by a cephalic bulb armed with 31–39 transverse rows of uncinated hooks, a comparatively long oesophagus, short spicules and the presence of a gubernaculum. The new species differs from its congeners by having four cervical sacs of almost equal length, a higher oesophagus/body length ratio, the arrangement of the caudal papillae, the absence of a medioventral preanal organ and numerous scattered `pores´ limited to the lateral side of the posterior part of the body. This is the first report of a member of the genus Echinocephalus Molin, 1858 from the Tunisian coast, and a new host and locality record for the Gnathostomatidae. A key to the species of Echinocephalus is provided.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Echinocephalus Molin, 1858 is a nematode genus whose members are found in the gastro-intestinal system of sharks and rays worldwide (Baylis & Lane, 1920; Soota, 1983; Moravec & Justine, 2006). The biology and life cycle of the species of Echinocephalus are poorly understood, although larval stages of this genus are known to infect sea urchins (Pearse & Timm, 1971), oyster (Cheng, 1975) and bivalves (Moazzam & Moazzam, 2014). Obligate intermediate hosts of Echinocephalus spp. are probably marine crustaceans (copepods), whereas sea urchins, bivalves and teleosts serve as paratenic hosts only (or possibly as the second intermediate hosts) (Ivashkin & Khromova, 1976; Anderson, 2000; Moravec & Justine, 2021). It has been postulated that Echinocephalus species can be considered zoonotic (Hoberg et al., 1998), possibly involving a health risk for humans (Miyazaki, 1960; Brooks & Deardorff, 1988; Jeremiah et al., 2011; Shamsi & Sheorey, 2018; Shamsi et al., 2021) when accidentally infected whilst consuming molluscs (Millemann, 1951, 1963). Ko et al. (1975) demonstrated that third stage larvae of E. crassostreai Cheng, 1975 [species inquirenda (Moravec & Justine, 2021)] from oysters, fed to a cat and a monkey penetrated the stomach and intestines of both animals.
The objective of this study is to describe a new species of Echinocephalus of the lesser spotted dogfish Scyliorhinus canicula off Kalaat El Andalous, NE Tunisia.
Materials & methods
Between 6th–23rd April 2019, 17 specimens of Scyliorhinus canicula were collected by fishermen from the Sea of Kalaat El Andalous, Gulf of Tunis (37°07'23.8"N 10°23'40.7"E), NE Tunisia. Fish were transported on ice directly to the Laboratory of Diversity, Management and Conservation of Biological Systems, Faculty of Sciences of Tunis, University of Tunis El Manar, Tunisia and studied for endohelminths. Fish nomenclature and classification followed Froese and Pauly (2020).
Six nematodes were found alive inside the spiral valve of the infected host, washed in physiological solution and stored in vials containing 70% ethanol. Morphometric studies were performed on 4 specimens (2 males and 2 females) while another 2 were used for Scanning Electron Microscopy (SEM). For light microscopic examination, 2 males and 2 females were cleared in lactophenol. Drawings were made with a drawing tube attached to an Olympus BX53 DIC microscope. For SEM, samples were dehydrated in an ascending series of ethanol and transferred to 100% acetone, critical point dried, mounted on aluminium SEM-carrier with adhesive conductive carbon tape (PLANO) and coated with gold (10 nm–20 nm layer) under vacuum (EM SCD 004, BALTE). Nematodes were analysed by a field emission scanning electron microscope (FE-SEM, MERLIN® VP Compact, Zeiss), and SEM-images were taken from the selected regions (conditions like applied detector, accelerating voltage, magnification, working distance are given in the figures). All measurements are in micrometers unless otherwise stated.
Results
Gnathostomatidae Railliet, 1895
Gnathostomatinae Railliet, 1895
Echinocephalus Molin, 1858 (type species: Echinocephalus uncinatus Molin, 1858; original designation)
Echinocephalus caniculus n. sp. (Figs. 1, 2, 3)
Echinocephalus caniculus n. sp. SEM micrographs of male (A): Anterior end, lateral view (scale bar: 50 µm); (B): Detail of interlabia (scale bar 5: µm); (C): Cephalic end, dorsoventral view (scale bar: 100 µm); (D): Deirid (scale bar: 5 µm); (E): Apical view of cephalic end/pseudolabia (asterisk: amphid, white arrow: cephalic papillae, black arrows: cuticular thickenings, stars: median lobe (black) and lateral lobes (white)) (scale bar: 20 µm); (F): Detail of amphid (asterisk) and cephalic papillae (arrow) (scale bar: 5 µm); (G): Rows of spines on cephalic bulb (scale bar: 5 µm); (H): Hooks (scale bar: 1 µm); (I): Posterior part of male, lateral view, numerous pores-like stricture (scale bar: 5 µm)
Echinocephalus caniculu n. sp. SEM micrographs of male (A): Posterior end, lateral view (scale bar: 100 µm); (B): Caudal papilla (scale bar: 5 µm); (C): Tail end, lateral view (scale bar 20: µm); (D&E): Posterior end, ventral view (scale bar: 100 µm); (F): Detail of papillae (scale bar: 20µm); (G): Small cuticular bosses on lateral side of cloaca (Area rugosa) (scale bar: 5 µm); (H): Caudal end, ventral view (scale bar: 20 µm). Symbols: Asterisk- phasmid, green arrow- precloacal and red arrows-postcloacal papillae
Type-host: Lesser spotted dogfish Scyliorhinus canicula (L.) (Carcharhiniformes: Scyliorhinidae).
Type-locality: Kalaat El Andalous, off the Mediterranean coast of North East Tunisia (37°07'23.8"N 10°23'40.7"E).
Site of infection: spiral valve.
Prevalence and intensity: 6% (1 infected/17 examined); 6 nematodes.
Type-material: Holotype (E.7649), allotype (E.7650) and paratypes [1 male (E.7651) and 1 female (E.7652)] from the same host and locality.
Depositories: holotype, allotype and 2 paratypes (male and female)—Berlin Natural History Museum, Germany.
Etymology: This species is named after its type-host species name, canicula where it was discovered and described for the first time.
Deposition of type specimens and Life Science Identifiers:
ZooBank registration:
In order to comply with the regulations, set out in article 8.5 of the amended 2012 version of the International Code of Zoological Nomenclature (ICZN, 2012), details of the new species have been submitted to ZooBank. The Life Science Identifier (LSID) for Echinocephalus caniculus n. sp. is urn:lsid:zoobank.org:pub:AFE7918E-1C87-4592-B9D4-492DD7B7A5B6.
Description
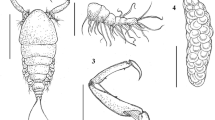
Body large, dark brown, unarmed except at cephalic bulb. Cuticle with fine transverse striation. Anterior extremity provided with 2 large, equal and massive lateral pseudolabia (Figs. 1A,B, 2A,C,E); each pseudolabium bearing 2 large sublateral/submedian double papillae and a lateral amphid (Figs. 2E,F). Pseudolabia trilobed (1 median, 1 dorsal and 1 ventral lobes); each lobe with cuticular thickenings along external surface (Fig. 2E). Each pseudolabium interlocking with opposite pseudolabium (Fig. 2E). A distinct cuticular striation observed on the dorsal and ventral parts of pseudolabia (Fig. 2B). Interlabia (1 dorsal and 1 ventral) small and serrated (Fig. 2B). Cephalic bulb prominent, armed with 31–39 transverse rows of small, elongated and evenly spaced, posteriorly directed uncinated spines; first few anterior rows of spines incomplete, situated close to each other and non-overlapping (Figs. 2A,G,H). Oesophagus long, not clearly divided into anterior muscular and posterior glandular portions (Fig. 1A). Cervical sacs four, almost equal in length, extending posteriorly and reaching about mid-length of oesophagus (Fig. 1A). Nerve ring and excretory pore not observed. Deirids prominent, well developed and located near posterior part of cephalic bulb (Figs. 1A, 2 C,D). At posterior part of body, numerous lateral pores or pit-like structures scattered in limited area (Fig. 2I). Phasmids paired, located laterally near end of tail (Figs. 1C,D, 3A,C). Tail conical with rounded tip; tip without mucron or any cuticular ornamentation in both sexes (Figs. 1C,D).
Male (paratype, measurements of holotype in parentheses)
Body length 26.74 (26.56) mm, maximum width 784 (816). Length of cephalic bulb 436 (531), width at middle part 684 (673). Oesophagus 5.90 (5.97) mm long (22–22% of body length). Cervical sacs 3.19–3.40 (3.19–3.42) mm long. Deirids 879 (1128) from anterior extremity. Spicules equal, 809 (897) long (3–3% of body length) and non-alate. Gubernaculum well-sclerotised, 163 long. Small caudal alae ventrolateral (Figs. 1C, 3A). On either side of cloaca, numerous small cuticular bosses (ornamentation) present (area rugosa not prominent) (Figs. 3F,G), ventral side smooth. Seven pairs of caudal papillae: three sub-ventral precloacal pairs and four pairs of postcloacal papillae of which three pairs subventral and one pair lateral (Figs. 3B,D,E). Caudal papillae not equidistantly distributed. First precloacal pair prominent, situated further away from second pair. A prominent second precloacal pair while third precloacal pair not-prominent situated near each other (Fig. 3F). Three postcloacal pairs close to each other just posterior to cloaca while last pair just in subventral position (Figs. 3F,H). Phasmids 181 from posterior end. Tail 577 long.
Gravid female (paratype, measurements of allotype in parentheses)
Body length 40.86 (30.18) mm, maximum width 849 (920). Cephalic bulb 465 (484) long, 751 (749) wide. Oesophagus 6.64 (6.50) mm long (16–21% of body length). Cervical sacs 2.84–3.14 mm long. Deirids 1.09 mm from anterior end. Vulva slightly elevated, located 1.8–2.4 mm from posterior end and at a short distance anterior of anus (Fig. 1D). Vagina anteriorly directed, open into uterus, divided into 2 branches. Uterus didelphic, both branches running parallel anteriorly (prodelphic). Eggs round to oval, thin-walled, 41 long and 33 wide (n= 5). Phasmids located laterally at 304 from posterior end.
Remarks
The present nematode species belongs to the genus Echinocephalus based on the unarmed body, two trilobed pseudolabia, a cephalic bulb provided with transverse rows of spines, a vulva close to the posterior end of the body and a didelphic, prodelphic uterus. So far, the taxonomy of this genus is incompletely resolved due to several incomplete descriptions. For example, six adult species of this genus namely; Echinocephalus chengii Singh, Chauhan & Khare, 2010, E. mastacembeli Begum & Gupta, 2012 (“it was apparently established on misidentified specimens of Spinitectus Fourment, 1884, probably S. mastacembeli Karve et Naik, 1951”), E. mobulae Kalyankar, 1971, E. scoliodonti Lakhsmi, 1994, E. unispiculus Arya, 1982 and E. waltairensis Lakshmi, Rao & Shyamasundari, 1984 have been reported in India (Moravec & Justine, 2021). However, because of their poor descriptions, they were designated as species inquirendae by Moravec and Justine (2021). Moreover, E. carpiae Abdel-Ghaffar, Bashtar, Mehlhorn, Abdel-Gaber, Al Quraishy and Saleh, 2013 and E. crassostreai were described based on their larval stages only (Cheng, 1975; Abdel-Ghaffar et al., 2013). Larval Echinocephalus have undeveloped morphological characteristics compared to adults, e.g., the number of cephalic hooks differs and the reproductive systems are not developed. Moravec and Justine (2021) did not consider E. carpiae and E. crassostreai as valid species but pointed out their stati as species inquirendae or species dubiae.
In terms of number of valid species, Bezerra et al. (2020) and Moravec and Justine (2021) recognised 10 and 12 Echinocephalus species respectively. Bezerra et al. (2020) did not consider E. daileyi Deardorff, Brooks & Thorson, 1981 and E. diazi Troncy 1969 as valid species while Moravec and Justine (2021) recognised both these species as valid.
As it possesses more than 30 transverse rows of hooks on the cephalic bulb, the present species differs from E. multidentatus Baylis & Lane, 1920, E. pseudouncinatus Millemann, 1951 and E. southwelli Baylis & Lane, 1920 which have 10–21 rows; and E. diazi, E. pteroplateae Wang et al., 1978 and E. sinensis Ko, 1975 which have 25–30 rows. Echinocephalus pteroplateae and E. sinensis have been reported from the same host order (Myliobatiformes) and zoogeographical region (China) and show similar numbers of transverse rows and length of body (Ko, 1975; Wang et al., 1978). There are minor variations in the distance between the nerve ring, oesophagi and deirids from the cephalic end (considered to be interspecific variation) while other data is not available for further comparison based on their poor descriptions. Therefore, we suggest that E. sinensis is a junior synonym of E. pteroplateae and, consequently, we have not included E. sinensis in our key to the species. However, Bezerra et al. (2020) and Moravec and Justine (2021) still considered E. pteroplateae and E. sinensis as separate species.
In possessing more than 30 transverse rows of hooks, the present species is similar to E. daileyi, E. janzeni Hoberg, Brooks & Urena, 1998, E. inserratus Moravec & Justine 2021, E. overstreeti Deardorff and Ko 1987, E. spinosissimus von Linstow, 1905 and E. uncinatus but differs in the combination of different morphological features, host and geographical location (see the key to species). The new species is different from E. daileyi (from freshwater stingrays, South America, Deardorff et al., 1981) and E. janzeni (from Pacific chupare, Styracura pacifica (Beebe & Tee-Van) Hoberg et al., 1998) as it has lower number of caudal papillae (7 pairs vs. 9 pairs), different hosts (Carcharhiniformes vs. Myliobatiformes) and localities (North Africa (Tunisia) vs. Central and South America).
By having interlabia with cuticular striations, the new species can be easily distinguished from E. inserratus. Moreover, the new species has been discovered in a different geographical location (North Africa vs. New Caledonia). The new species differs from E. spinosissimus by having a lower number of caudal papillae (7 pairs vs. 8 pairs) and a longer distance between the vulva and the tip of the tail (1.8-2.4 mm vs. 1.1-1.4 mm). Similarly, the new species is reported from a different location to E. spinosissimus (North Africa vs. Indian Ocean) (Baylis & Dubney, 1920; Beveridge, 1985). By having equal spicules and lower numbers of cloacal papillae, the new species can be differentiated from E. uncinatus which has unequal spicules and a higher number of cloacal papillae. Again, the new species has been found in different region (North Africa vs. North Atlantic region) (Beveridge, 1985).
Echinocephalus overstreeti most closely resembles the new species but differs in that it has cervical sacs of almost equal length of (vs. unequal), a higher oesophagus-body length ratio (16–22% vs. 11–16%), a ventral surface of the male tail (annulations vs. smooth) and reported different hosts and localities (see the key to species) (Deardorff and Ko, 1983; Beveridge, 1987; Moravec & Justine 2006). Besides, E caniculus n. sp. differs in possessing numerous pore-like structures scattered laterally on the posterior part of the body.
The new species is also compared with those species that have been reported from similar geographical regions. E. spinosissimus has been recorded only from the Adriatic Sea (Baylis & Lane, 1920), which is in a similar geographical region to our new species. As mentioned above, E. caniculus n. sp. is distinct from E. spinosissimus.
Echinocephalus carpiae [species inquirenda], E. janzeni, E. inserraus, E. overstreeti and E. sinensis [junior syn. of E. pteroplateae] have been studied by scanning electron microscopy (Hoberg et al., 1998; Moravec & Justine, 2006, 2021; Abdel-Ghaffar et al., 2013), illustrating these specimens more precisely. We conclude that there are 12 valid species in the genus Echinocephalus and the key to each species is provided below.
Key to species of the genus Echinocephalus Molin, 1858
-
1a
Cephalic bulb with 10-21 transverse rows of hooks…2
-
1b
Cephalic bulb with more than 25 transverse rows of hooks…3
-
2a
11-13 transverse rows of hooks, 8 pairs of caudal papillae, vulva 0.6-0.65 mm from posterior end, from Indian Ocean (Ceylon), Myliobatiformes…E. multidentatus
-
2b
14-21 transverse rows of hooks…4
-
3a
25-30 transverse rows…5
-
3b
More than 30 transverse rows…6
-
4a
16-21 transverse rows, 6 pairs of caudal papillae, vulva 2.53-3.52 mm from posterior end, from South America (Mexico), Heterodontiformes…E. pseudouncinatus
-
4b
15-18 transverse rows, 8 pairs of caudal papillae, vulva 0.55 mm from posterior end, from Indian Ocean (Ceylon), Myliobatiformes…E. southwelli
-
5a
5-7 pairs of caudal papillae, Oe/BL ratio 15-20%, from Southern China and New Caledonia, Myliobatiformes…E. pteroplateae
-
5b
8 pairs of caudal papillae, Oe/BL ratio 8-12%, from Venezuela, Myliobatiformes…E. diazi
-
6a
7 pairs of caudal papillae (rarely 8)…7
-
6b
8-9 pairs of caudal papillae…8
-
7a
Cervical sac of unequal size, 7 (rarely 8) pairs of caudal papillae, Oe/BL ratio 11-16%, spicules unequal, spicules/BL ratio 2.0-5.0%, gubernaculum present, from Australia, French Polynesia and New Caledonia, Heterodontiformes, Myliobatiformes, Orectolobiformes, Rajiformes, Rhinopristiformes, Torpediniformes and Chimaeriformes…E. overstreeti
-
7b
Cervical sacs of equal size, 7 pairs of cervical papillae…9
-
8a
8 pairs of caudal papillae…10
-
8b
9 pairs of cervical papillae…11
-
9a
Interlabia with distinct cuticular striation, 7 pairs of caudal papillae, Oe/BL ratio 16-22%, Spicules/BL ratio 3.0-3.1%, Gubernaculum present, from Tunisia, Carcharhiniformes…E. caniculus n. sp.
-
9b
Interlabia without distinct cuticular striation, 7 pairs of caudal papillae, Oe/BL ratio 17-20%, spicules almost equal, spicules/BL ratio 4.5-9.2%, Gubernaculum present, from New Caledonia, Myliobatiformes…E. inserratus
-
10a
8 pairs of caudal papillae, spicules/BL ratio 10-14.3% (in contrast to 7a), spicules unequal, area rugosa present, vulva 1.4-2.5 mm from posterior end, from Adriatic and Black Sea, Myliobatiformes…E. uncinatus
-
10b
8 pairs of caudal papillae, spicules/BL ratio 6.6-7.4% (in contrast to 7a), spicules equal, area rugosa absent, vulva 1.1-1.4 mm from posterior end, from Indian Ocean, Myliobatiformes…E. spinosissimus
-
11a
9 pairs of caudal papillae, body length 55-65mm, spicules equal, from Central and South America, Myliobatiformes…E. daileyi
-
11b
9 pairs of caudal papillae, body length 13-33mm, spicules unequal, reported from Central and South America, Myliobatiformes…E. janzeni
Conclusion
By comparing the morphology of adult male and female Echinocephalus specimens from Scyliorhinus canicula collected off Tunisia with the descriptions of all congeners, it is evident that the collected specimens represent a new species, E. caniculus n. sp. The morphological description with notes on ecology, host specificity and zoogeography add new information to the knowledge of the parasite fauna of the lesser spotted dogfish in general, and in the Mediterranean basin sensu stricto, providing the first record of nematodes of the genus Echinocephalus in Tunisia.
Availability of data and material
All data published within this text.
Code availability
Not applicable
References
Abdel-Ghaffar, F., Bashtar, A. R., Mehlhorn, H., Abdel-Gaber, R., Al Quraishy, S., & Saleh, R. (2013). Morphological and phylogenetic analysis of Echinocephalus carpiae n. sp. (Nematoda: Gnathostomatidae) infecting the Common carp Cyprinus carpio inhabiting Burullus Lake - a new host record in Egypt. Parasitology Research, 112(12), 4021-4028.
Anderson, R. C. (2000). Nematode Parasites of Vertebrates. Their Development and Transmission. CAB International.
Baylis, H. A., & Lane, C. (1920). A revision of the nematode family Gnathostomidae. Proceedings of the Zoological Society of London, 90(3), 245-310.
Beveridge, I. (1985). A redescription of Echinocephalus uncinatus Molin, 1858 (Nematoda, Gnathostomatoidea) from European rays, Dasyatis pastinaca (Linnaeus, 1758). Bulletin du Muséum national d'histoire naturelle. Section A, Zoologie, Biologie et Ecologie Animales, 7(4), 781-790.
Beveridge, I. (1987). Echinocephalus overstreeti Deardorff & Ko, 1983 (Nematoda: Gnathostomatoidea) from elasmobranchs and molluscs in South Australia. Transactions of the Royal Society of South Australia, 111, 79-92.
Bezerra, T. N., Decraemer, W., Eisendle-Flöckner, U., Hodda, M., Holovachov, O., Leduc, D., Miljutin, D., Mokievs.ky, V., Peña Santiago, R., Sharma, J., Smol, N., Tchesunov, A., Venekey, V., Zhao, Z., & Vanreusel, A. (2020). Nemys: World Database of Nematodes. Echinocephalus Molin, 1858. World Register of Marine Species. Retrieved December 11, 2020, from http://www.marinespecies.org/aphia.php?p=taxdetails&id=22883.
Brooks, D. R., Deardorff, T. L. (1988). Rhinebothrium devaneyi n. sp. (Eucestoda: Tetraphyllidea) and Echinocephalus overstreeti Deardorff and Ko, 1983 (Nematoda: Gnathostomatidae) in a Thorny back ray, Urogymnus asperrimus, from Enewetak Atoll, with phylogenetic analysis of both species groups. Journal of Parasitology, 74(3), 459-465.
Cheng, T. C. (1975). Echinocephalus crassostreai sp. nov., a larval nematode from the oyster Crassostrea gigas in the Orient. Journal of invertebrate pathology, 26(1), 81-90.
Deardorff, T. L., Brooks, D. R., & Thorson, T. B. (1981). A new species of Echinocephalus (Nematoda: Gnathostomidae) from Neotropical stingrays with comments on E. diazi. Journal of Parasitology, 67(3), 433-439.
Froese, R., & Pauly, D. (2020). FishBase. World Wide Web electronic publication. Retrieved November 15, 2020 from http://www.fishbase.org.
Hoberg, E. P., Brooks, D. R., Molina-Ureña, H., & Erbe, E. (1998). Echinocephalus janzeni n. sp. (Nematoda: Gnathostomatidae) in Himantura pacifica (Chondrichthyes: Myliobatiformes) from the Pacific coast of Costa Rica and Mexico, with historical biogeographic analysis of the genus. Journal of Parasitology, 84(3), 571-581.
ICZN. (2012). International Commission on Zoological Nomenclature: Amendment of articles 8, 9, 10, 21 and 78 of the International Code of Zoological Nomenclature to expand and refine methods of publication. Bulletin of Zoological Nomenclature, 69, 161-169.
Ivashkin, V. M., & Khromova, L. A. (1976). Cucullanata and Gnathostomatata of Animals and Man and the Diseases Caused by Them. Osnovy Nematodologii 27. Nauka (In Russian.)
Jeremiah, C. J., Harangozo, C. S., & Fuller, A. J. (2011). Gnathostomiasis in remote northern Western Australia: the first confirmed cases acquired in Australia. The Medical Journal of Australia, 195(1), 42-44.
Ko, R. C. (1975). Echinocephalus sinensis n. sp. (Nematoda: Gnathostomatidae) from the ray (Aetabatus flagellum) in Hong Kong, southern China. Canadian Journal of Zoology, 53(4), 490-500.
Ko, R. C., Morton, B., & Wong, P. S. (1975). Prevalence and histopathology of Echinocephalus sinensis (Nematoda: Gnathostomatidae) in natural and experimental hosts. Canadian Journal of Zoology, 53(5), 550-559.
Millemann, R. E. (1951). Echinocephalus pseudouncinatus n. sp., a nematode parasite of the abalone. Journal of Parasitology, 37(5), 435-439.
Millemann, R. E. (1963). Studies on the taxonomy and life history of echinocephalid worms (Nematoda: Spiruroidea) with a complete description of Echinocephalus pseudouncinatus Millemann, 1951. Journal of Parasitology, 49(5), 754-764.
Miyazaki, I. (1960). On the genus Gnathostoma and human gnathostomiasis, with special reference to Japan. Experimental Parasitology, 9(3), 338-370.
Moazzam, M., & Moazzam, N. (2014). Some observations on the infestation of bivalve Scapharca natalensis by larval nematode Echinocephalus (Nematoda: Gnathostomatidae). Pakistan Journal of Zoology, 46(5), 1455-1458.
Moravec, F., & Justine, J. L. (2006). Three nematode species from elasmobranchs off New Caledonia. Systematic Parasitology, 64(2), 131-145.
Moravec, F., & Justine, J. L. (2021). Echinocephalus inserratus sp. n. (Nematoda: Gnathostomatidae) from the stingray Pastinachus ater (Dasyatidae) and new records of congeneric and some other nematode larvae from teleost fishes off New Caledonia. Folia Parasitologica, 68, 014.
Pearse, J., & Timm, R. (1971). Juvenile nematodes (Echinocephalus pseudouncinatus) in the gonads of sea urchins (Centrostephanus coronatus) and their effect on host gametogenesis. Biological Bulletin, 140(1), 95-103.
Shamsi, S., & Sheorey, H. (2018). Seafood‐borne parasitic diseases in Australia: are they rare or underdiagnosed? Internal Medicine Journal, 48(5), 591-596.
Shamsi, S., Steller, E., & Zhu, X. (2021) .The occurrence and clinical importance of infectious stage of Echinocephalus (Nematoda: Gnathostomidae) larvae in selected Australian edible fish. Parasitology International, 83, 1-7.
Soota, T. (1983). Studies on nematode parasites of Indian vertebrates. 1. Fishes. Zoological Survey of India.
Wang, P. Q., Zhao, Y. R., & Chen, Q. Q. (1978). Report on some nematodes from vertebrate animals in South China. Journal of Fujian Teachers University (Natural Science), 2, 75–90.
Acknowledgements
We thank Dr. Markus Frank, Dr. Armin Springer and Mrs. Karoline Schulz from University Medicine, Medical Biology, Electron Microscopy Center, Rostock University, Germany for their help and kindness with obtaining SEM micrographs. Prof. Juan T. Timi (Ichthyoparasitology Laboratory, Institute of Marine and Coastal Research, Faculty of Science, National University of Mar del Plata, Argentina) for helping us with taxonomy and for his valuable suggestions and comments to the first version of our draft. In addition, we would like to thank Dr. Sarah H. Al-Jufaili (Ministry of Fisheries Wealth, Fishery Quality Control Centre, Al Bustan-Muscat, Oman) for helping with image processing with Adobe Photoshop.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was funded by the Ministry of Higher Education and Scientific Research of Tunisia, University of Tunis El Manar, LR18ES06 Tunis, Tunisia, Faculty of Sciences of Tunis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Research involving human participants and/or animals
No alive marine fish was treated or killed for the purpose of the present research, but were collected after their death for human consumption. All applicable international, national and/or institutional guidelines for the care and use of animals were followed. Fish species is not listed in CITES or CMS and listed under Least Concern in IUCN Red List Status.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Saad, C.B., Suthar, J., Theisen, S. et al. Echinocephalus caniculus n. sp. (Nematoda: Gnathostomatidae Railliet, 1895) from the lesser spotted dogfish Scyliorhinus canicula (L.) (Elasmobranchii: Scyliorhinidae Gill, 1862) off Tunisia, with a key to species of the genus Echinocephalus. Syst Parasitol 99, 299–307 (2022). https://doi.org/10.1007/s11230-022-10027-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11230-022-10027-7