Abstract
This paper critically examines the philosophical underpinnings of current experimental investigation into animal affect-related decision-making. Animals’ affective states are standardly operationalised by linking positively valenced states with “approach” behaviours and negatively valenced states with “avoidance” behaviours. While this operationalisation has provided a helpful starting point to investigate the ecological role of animals’ internal states, there is extensive evidence that valenced and motivational states do not always neatly align, namely, instances where “liking” does not entail “wanting” (and vice versa). To address this limitation, this paper proposes the (re-)integration of dominance as a dimension of affect. In particular, I argue that dominance, construed as a measure of organisms’ self-assessed behavioural control, can provide the necessary nuance to understand animal affect-related decision-making. Thus, after addressing the theoretical and methodological challenges related to incorporating dominance into a definition of “core affect”, this paper introduces the “Valence-Arousal-Dominance” model of animal affect. This model is explored for its potential applications in two domains. Firstly, in the study of animal affect-related decision-making under predation risk, and secondly, in the study of animal wellbeing. Through these applications, this model aims to bring experimental paradigms of animal affect-related behaviour closer to ecologically relevant scenarios.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Affective states, such as emotions and moods, constitute a thriving area of research within the field of non-human animal (henceforth “animal”) behavioural biology (Mellor, 2016; Mendl & Paul, 2020; Neethirajan et al., 2021). This interest stems from the increasing evidence indicating that positively and negatively valenced affective states exert a pivotal influence on animals’ cognitive landscape and well-being (Webber et al., 2022). Despite standard scepticism regarding the explanatory value of affective states in ethological research (Burghardt, 2019), growing interest in the ecological consequences of individual differences in behaviour, especially those linked to affect-driven decision-making, underscores the significance of investigating the emotional aspects of animals’ life (Laskowski et al., 2022).
In psychology, speech reports are usually employed as a benchmark for inferring human emotional experiences and measuring their subjective or “conscious” quality. Due to the absence of such indicators in animals, biological research primarily investigates animal emotions by focusing on their underlying mechanisms and functions instead (Paul et al., 2005). That is, the mechanisms by which emotions assist organisms in coordinating different resources to navigate environmental challenges efficiently. When faced with a threat, fear prompts organisms to fight or flee, modulates their heart rate, and sharpens their attention. Thus, despite the absence of direct evidence of animals’ conscious experience of affective states, investigating their affective states proceeds by measuring their putative behavioural, physiological, and cognitive components.
Within this endeavour, researchers typically delineate the contrast between positively and negatively valenced states in terms of opposite motivational tendencies, where positive states prompt approach and negative states elicit aversion towards the state’s object (Lang, 1995). This assumption has been particularly prominent within the Judgment Bias Test (JBT), which explores the influence of affective states on decision-making by measuring animals’ responses to ambiguous stimuli (Harding et al., 2004; Mendl et al., 2010). In a standard JBT, animals initially learn to respond differently to two distinct cues that signal a positive or a negative outcome (e.g., food rewards or punishments, respectively). Subsequently, when confronted with intermediate, ambiguous cues, their behavioural responses—approaching or avoiding such cues—serve as indicators of the organism’s relative degree of bias to judge them as expecting rewards (“optimism”) or punishments (“pessimism”).
This framework has facilitated cross-species comparison of affect-related decision-making, spanning mammals, fish, and insects, thereby offering valuable insights into ultimate questions regarding the evolutionary significance of affective states (Lagisz et al., 2020). However, it bears some limitations. Outside the laboratory, an event’s outcomes often defy neat categorisation as purely rewarding or punishing. Confronted with such events, foraging organisms may judge potential energy gains more valuable than safety, leading them to seek rewards and thus display a “risk-prone” behaviour. Conversely, they may consider safety more valuable than energy gains and consequently attempt to avoid such events, displaying “risk-averse” behaviour. According to the framework outlined above, risk-prone behaviour would then be associated with a positive state, reflecting an overall evaluation of the situation as rewarding. However, risk-prone behaviour has also been observed to correlate with anxiety and aggressiveness (Sih et al., 2004), which are states that cannot be described as positive.
This case, along with extensive evidence indicating that “liking” and “wanting” a stimulus do not always converge (to be reviewed in Sect. 2.3), calls for a more nuanced framework to investigate animals’ affect-related decision-making. In pursuit of this goal, this paper defends a new operational definition of affective states that introduces a conative dimension: “dominance”. The basic idea is that, whereas the activation of valence is influenced by how rewarding or punishing an environment is, the activation of dominance involves how capable individuals perceive themselves to be in obtaining such rewards or overcoming such punishments. Although early dimensional models of affect featured such a conative dimension (Osgood et al., 1957; Mehrabian & Russell, 1974), these were later considered peripheral to “core affect”, namely, valence and arousal (Russell & Barrett, 1998; Barrett & Russell, 1999). In response, this paper advocates for defining dominance as a measurement of the organism’s subjective experience of behavioural self-control vis-à-vis environmental challenges and addresses the criticisms against its inclusion in the definition of core affect (Sect. 3).
Then, drawing inspiration from Mendl et al. (2010), this paper develops a behavioural operationalisation of the “Valence-Arousal-Dominance” framework (Sect. 4). The framework is explored for its potential applications in two domains. Firstly, I argue that the interplay between valence and dominance can offer insights into state-of-the-art experimental research on animal risk-taking behaviour, drawing an example from Bračić et al. (2023) (Sect. 5). Secondly, I explore how dominance might elucidate not only the influence of emotions on animals’ survival but also on their thriving. Inspired by Hintze and Yee (2023), I link dominance to the study of “flow” in animals, namely, a state of absorption that requires the equilibrium between individual capabilities and environmental challenges (Sect. 6). Through these applications, this model aims to bring experimental paradigms of animal affect-related behaviour closer to ecologically relevant scenarios.
2 Background: the study of animal affect-related decision-making
2.1 Varieties and models of affect
Affective states come in various types. Emotions like joy or anger are standardly considered states directed towards particular stimuli provided by their cognitive bases (e.g., perception or memory) (Kenny, 1963). In contrast, moods such as grumpiness or elation are considered relatively enduring states that lack a specific object and that trigger emotions of the same affective colour (and vice-versa) (Frijda, 1994a). Being grumpy, for instance, may incite an organism to feel angry, and feeling angry may induce a state of grumpiness. Alternative taxonomies of affective phenomena also include personality or “character” traits such as boldness, aggressiveness, or optimism. Like moods, traits are said to motivate particular reactions: if someone is aggressive, for instance, they will display a greater than typical tendency to feel, say, anger or anxiety. However, in contrast to moods, character traits lack a subjective quality, except derivatively through the affective states in which they find expression (Deonna & Teroni, 2012).
When investigating these phenomena, affective states are conceptualised through discrete or dimensional models. Discrete models conceptualise emotions, such as anger or fear, as natural kinds with specific behavioural, physiological, and cognitive fingerprints (Ekman, 1984; Panksepp, 1998). For instance, Panksepp’s (1998) research on “genetically ingrained brain emotional operating systems”, which he marks with capitalised terms such as RAGE or FEAR, has revealed various similarities in their underlying neuronal bases across mammals. However, the search for emotions’ distinctive patterns has yielded mixed results. Critics point out, for example, that different responses (e.g., different patterns of autonomic activation) can be associated with a single emotion type and that, conversely, different emotion types (e.g., anger and joy) can be associated with similar physiological responses, raising scepticism about whether different types of emotions share intrinsic properties (Barrett, 2017).
Alternatively, dimensional models view affective states as composed of more fundamental building blocks. One prominent model in this category is the “Circumplex Model” (Russell, 1980). In contrast to the discrete model, which posits that independent neuronal systems subserve each emotion type, the circumplex model proposes that all affective states arise from two neurophysiological systems. One system is related to valence—the “hedonic” quality of the state, ranging from negative to positive—while the other is related to arousal—the degree of physiological activation, ranging from low to high. Specific affective states can be thus located along two orthogonal dimensions, with the X-axis representing the degree of valence and the Y-axis representing the degree of arousal. For instance, in this framework, “Happy” and “Content” would arise from the same two neurophysiological systems but differ in their degree of arousal (see Fig. 1). While analysing animals’ discrete states has traditionally received more attention (see, for instance, De Waal and Preston (2017) on animal empathy), the dimensional perspective has gained prominence within behavioural biology, as we will explore in the next section.
2.2 Circumplexity and the Judgment Bias Test
Research in human psychology suggests that alterations in information processing serve as reliable indicators of individuals’ emotional states (MacLeod & Byrne, 1996). People undergoing negative states, such as depression or anxiety, tend to make more negative judgments about ambiguous stimuli relative to their counterparts in happier states. Recently, these findings have sparked interest in whether such affect-induced biases exist in animals, mainly through the judgment bias paradigm (Harding et al., 2004; Mendl et al., 2010). This section describes how the circumplex model is employed within this paradigm.
Mendl et al. (2010) propose to associate the transition from non-arousing, negative states to highly arousing, positive states with the activation of the “reward acquisition” system (RAS). Conversely, the shift from non-arousing, positive states to highly arousing negative states is proposed to involve the “punishment-avoidance” system (PAS) (see also Mendl & Paul, 2020). These “biobehavioural mechanisms” are assumed to guide the organism in obtaining fitness-enhancing rewards (e.g., food, shelter, etc.) or avoiding exposure to fitness-threatening punishers (e.g., predator attack, thermal damage, etc.) (Watson et al., 1999). Figure 1 illustrates the resulting functional interpretation of the structure of core affect:
Functional operationalisation of the Circumplex Model, adapted from Mendl et al. (2010). The X-axis represents the valence dimension, and the Y-axis represents the arousal dimension. Words in italics indicate the location of specific affective states, including emotions (“Fearful”) and moods (“Anxious”). Arrows indicate the hypothetical link between the reward acquisition system and the Q3-Q1 axis (green) and the punishment avoidance system and the Q2-Q4 axis (orange)
Grounded on evidence that judgment bias in humans may indicate their positive or negative states, the framework proceeds to map the Q1-Q4 states with types of decision-making. Q1 states are postulated to trigger decisions appropriate to high reward-opportunity environments and thus to reflect individuals’ high expectation of positive events, and Q2 moods to trigger decisions reflecting low expectation of adverse events (“optimistic biases”). Conversely, Q3 moods trigger decisions associated with low expectations of positive events and Q4 moods trigger decisions associated with high expectations of adverse events (“pessimistic biases”).
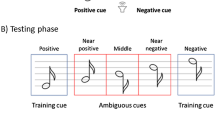
Based on these assumptions, the Judgment Bias Test typically comprises the following three phases (see Fig. 2):
-
A.
During the training phase, animals are presented with “positive” cues, which result in a reward, and “negative” cues, which result in a punishment (e.g., white noise). Through repeated trial and error, animals learn to respond positively by approaching the reward-associated stimuli and negatively by avoiding the punishment-associated stimuli. That is, by activating the RAS and PAS, respectively.
-
B.
During the testing phase, animals are periodically exposed to cues which are qualitatively “ambiguous” with respect to the training positive and negative cues (e.g., sounds of intermediate frequency with respect to training cues). Importantly, the behavioural responses to the ambiguous cues are considered indicative of animals’ expectations of positive or negative outcomes, operationalised as “optimistic” or “pessimistic” biases, and thus of their location in the valence-arousal space (Q1-Q4).
-
C.
During the priming phase, a subset of animals undergoes a treatment intended to alter their affective state (their mood) either positively (e.g., clean bedding) or negatively (e.g., unpredictable housing) before the testing phase. In contrast, the other animals serve as the control group.
Example of the JBT design in Nematipour et al. (2022), representing (A) the training phase, where animals are presented with low and high-pitched tones; (B) the testing phase, where animals are also presented with pitches intermediate to the training tones; and (C) the priming phase, where a subgroup of the animals is exposed to unpredictable housing to induce a negative state before testing
In analysing the outcomes of a JBT with negative priming, researchers have found significant differences between treatment and control groups across species (Lagisz et al., 2020). In particular, animals undergoing a treatment hypothesised to trigger negative states tend to exhibit a pessimistic bias by responding more often in a negative way to ambiguous cues compared to those of the control group. These results not only demonstrate the paradigm’s ability to detect changes in affective states but also provide evidence that the applied priming manipulation has a lasting impact on the animals’ positive or negative states. Hence, they illustrate the valence-arousal model’s ability to generate testable hypotheses concerning animals’ emotional experiences.
2.3 Issues in bi-dimensional affect: distinguishing “liking” from “wanting”
While this bi-dimensional model provides a valuable foundation for empirically investigating affect’s influence on animals’ cognition, it encounters various limitations. As observed, the model aligns affective states with systems governing approach and avoidance behaviours, positioned at a 45º angle to the core affect axes (see Fig. 1). However, this assumption is not straightforwardly valid. In humans, addictive disorders involve seeking stimuli even in the absence of pleasure (Becker et al., 2009). In animals, “predator inspection” involves approaching potentially dangerous stimuli (Blaszczyk, 2017). Using Becker et al.’s (2009) terminology, “wanting” and “liking” a stimulus sometimes dissociate from each other.
This dissociation not only represents a deviation from the norm but is observable in some animals’ common affairs. Within the JBT, animals’ behavioural responses indicate their attitudes towards stimuli that may be rewarding or punishing, but not both. However, during foraging under predation risk, animals often find themselves in situations that encompass both rewarding and punishing aspects, such as challenging-to-access food resources. In such situations, organisms may prioritise energy gains over safety, displaying risk-prone behaviours, or prioritise safety over energy gains, displaying risk-averse behaviours. Risk-prone behaviours entail seeking and thus approaching a stimulus. Hence, based on the framework outlined in Sect. 2.2, these behaviours might be construed as a proxy for organisms’ positively valenced states. However, this would be inaccurate: risk-prone dispositions also appear to correlate with anxiety and aggressiveness (Sih et al., 2004), suggesting that pleasure and motivational tendencies do not typically align.
A standard proposal for characterising risk-related attitudes posits that organisms rank their needs and motivations using valence as a “common” currency (Cabanac, 1992). When faced with conflicting motivations, organisms would aim to select the option that maximises pleasure. In scenarios where accessing food involves facing threats, for instance, animals would assess potential energy gains against the risk of predation based on their current energy balance, ultimately deciding on an “overall” evaluative response (e.g., approaching the stimulus despite the threat of predation due to the organism’s hunger). Hence, in the common currency account, we do not need to abandon the assumption that positive [negative] polarity aligns with approaching [avoiding] behaviours. Positive states would trigger an approach only in cases where it is, overall, stronger than negative states.
Recent research has provided compelling experimental evidence of this “expected utility” approach to animal decision-making (Verdolin, 2006). However, many studies have also failed to demonstrate optimal foraging behaviour under predator risk. The likelihood of a higher number of negative results exists, mainly due to general publication bias against negative findings (Dammhahn & Almeling, 2012). Certain organisms exhibit risk-prone or risk-averse behaviour regardless of their energy budget. Consequently, risk-taking can only partially be attributed to optimal trade-off calculations regarding the valence of different stimuli. The presence of sub-optimal animal risk-taking behaviour underscores the necessity of exploring additional factors that may influence animals’ decision-making.
A prominent approach for investigating sub-optimal behaviour involves assessing animals’ personalities. In the biological context, personality is defined as interindividual consistent variation in behavioural traits (Sih et al., 2004; Réale et al., 2007). For instance, stable individual differences in risk-taking behaviour are termed “boldness”, hypothesised to be an essential source of variation constraining decision-making. Importantly, as seen in Sect. 2.1, personality is assumed to interact with organisms’ affective states, constraining their behavioural flexibility. In support of this claim, there is evidence that both an organism’s personality and mood influence its cognitive biases (Asher et al., 2016). Therefore, it is crucial to study risk-taking behaviour not only by considering organisms’ personality traits but also the emotions these traits may trigger. Achieving this requires a novel framework of affect that clearly distinguishes valence from motivation. In what follows, I argue that adding a conative dimension beyond valence and arousal provides the required nuance.
3 Introducing the dominance dimension
3.1 Three theoretical proposals
In this section, I briefly contrast three strategies to enhance dimensional models of affect. First, to distinguish qualitatively different states, constructivist theories incorporate a categorisation process between the experience of affect and its outcomes (Barrett, 2017). Within this perspective, emotions emerge from individuals’ conceptualisations of their sensations of valence and arousal. Accordingly, labels such as “anger” and “fear” denote different concepts that individuals apply to their heightened negative sensations based on contextual factors. Nevertheless, while it is standardly agreed that affective states possess cognitive underpinnings (Deonna & Teroni, 2012), it is unclear whether and how animals would acquire the conceptual knowledge believed to ground their emotional experiences (Scherer, 2009).
Second, appraisal approaches to emotions distinguish between emotional states by interposing a stimulus evaluation process between what is perceived and the affective experience (Ellsworth & Scherer, 2003). To the extent that appraisals play a crucial role in characterising emotions, these theories are thus compatible with a dimensional view of affect. Thus, the qualitative difference between overlapping states in the valence-arousal space would result from the type of appraisal giving rise to each. For instance, fear and anger are respectively caused by assessing the triggering stimulus as dangerous and offensive. However, despite the potential of exploring appraisal processes in animal behaviour research, the appraisal theory primarily focuses on emotions. Instead, we require a comprehensive framework encompassing moods and, if possible, attitudes such as optimism.
Third, we may consider that “core affect” is not limited to valence and arousalf. Early dimensional models of affect often included a third, “conative” dimension. These models were first introduced within the so-called “Semantic Differential Technique” (SDT) framework, which measured the affective meaning of emotion-related words and other stimuli using bipolar scales (Osgood et al., 1957). Mehrabian & Russell (1974), for instance, proposed the “Pleasure-Arousal-Dominance” (PAD) model to study the degree to which individuals perceived a stimulus as pleasant (positive-negative), arousing (high-low) and dominant (dominant-submissive). The PAD model soon extended its scope of application, encompassing the development of metrics for emotions (Russell & Mehrabian, 1977) and personality traits (Mehrabian, 1996). As an illustration, consider Mehrabian’s (1996) model, depicted in Fig. 3. Note that, although the model is designed to distinguish between personality traits, the states it represents can be described as moods (e.g., “Relaxed”) or emotions (e.g., “Hostile”):
PAD model’s space of personality traits adapted from Mehrabian (1996)
The addition of a conative dimension can allow us to investigate decision-making in risk-taking contexts. However, various obstacles need to be surmounted before applying such a model. First, while dominance was described through expressions such as “controlling”, “influential”, or “autonomous” (Mehrabian & Russell, 1974; Russell & Mehrabian, 1977), a clear definition of it was missing (Bakker et al., 2014). Second, within SDT studies, stimuli ratings along dominance were not always independent of the other two dimensions (Russell & Mehrabian, 1977). Due to these findings, current dimensional models redefine dominance as denoting assessments of the antecedents and consequences of an affective experience, thereby acknowledging it as a peripheral cognitive process (Russell, 1978; Russell & Barrett, 1998). In what follows, I address these two issues in detail.
3.2 Dominance as (self-)control
The label “dominance” has many usages in ordinary language and has been used in different ways within dimensional models of affect (Bakker et al., 2014). Hence, before discussing whether dominance may or may not pertain to core affect, we need to put the concept of dominance on better theoretical grounds. The psychological literature on (self-)control, I think, offers a robust foundation for defining dominance. To begin with, Gregory (1978) proposed that individuals evaluate control separately of whether the events are appraised as positive or negative. According to him, regulating positive events entails attaining a positive reinforcer, while regulating adverse events involves avoiding aversive circumstances. Later on, Rothbaum, Wisz, and Snyder (1982) argued that individuals can evaluate their control in response to events in two different ways: “primary control”, which pertains to attempts to align events with one’s desires, and “secondary control”, which involves attempts to adapt oneself to environmental influences.
Based on these insights, Bryant (1989) examined the interplay between primary/secondary control and positive/negative evaluations. As a result, he proposed a four-factor model of perceived control, encompassing individuals’ self-evaluations of their ability to (a) avoid adverse events (“primary negative control”), (b) cope with adverse events (“secondary negative control”), (c) obtain positive events (“primary positive control”) and (d) savour those events (“secondary positive control”). Bryant hypothesised that primary control arises from attitudes regarding behavioural control over aversive or positive events, while secondary control arises from the strategies individuals use to regulate their emotions, either by coping with or prolonging them. Hence, while primary control mainly operates on the anticipation of future outcomes, secondary control pertains to the management of prior events.
As we are currently investigating animals’ affect-related decision-making, our focus will be on primary control. Therefore, I define dominance as a parameter related to an individual’s inherent drive or inclination as determined by their self-assessed behavioural capacity to obtain positive events and avoid negative ones. Thus, whereas valence, the hedonic quality of an affective experience, is influenced by an individual’s attitude towards the likelihood of a stimulus being positive or negative, dominance is based on an individual’s assessments of their capability to secure the former and evade the latter. This self-assessment spans a spectrum from low to high: individuals can assess themselves as highly or lowly capable of obtaining [avoiding] a positive [negative] outcome. Additionally, I consider that such an assessment can be intrinsically oriented (that is, an assessment of one’s capacity to temper with nature) or, on the flip side, externally oriented (that is, an assessment of whether nature is manageable, also termed “risk-perception”, see Hansson, 2023).Footnote 1
3.3 Rethinking core affect
Having defined dominance, I turn to the empirical, conceptual, and methodological issues regarding its inclusion in the definition of core affect. First, as observed above, individuals struggled to rate stimuli (e.g., isolated words) along a dominance scale (Russell & Mehrabian, 1977). However, more recent studies show that individuals do indeed factor into the dominance dimension when assessing other types of stimuli, such as facial expressions (Oosterhof & Todorov, 2008) or social interactions (Moors & De Houwer, 2005). This discrepancy in the relevance of dominance may thus be attributed to the type of stimuli used within these studies. Hence, relying on verbal reports may not be enough to settle theoretical debates about affect’s dimensions (Harmon-Jones et al., 2017).
Second, the rationale behind the location of dominance as a cognitive process and thus peripheral to core affect bears some conceptual shortcomings, as researchers nowadays acknowledge that the cognitive/non-cognitive demarcation is not as clear-cut as standardly considered. In philosophy, emotions are described not only as having cognitive bases (that is, as anchored on perception or memory) but also as possessing representational properties (that is, as qualifying stimuli and being subject to rational justification) (Scarantino & de Sousa, 2018). In some versions of the appraisal theory in psychology (introduced in Sect. 3.1), cognitive assessments of stimuli are not considered causes of affective states but part of the experience of affect itself. Lastly, within evolutionary psychology, it is standardly considered that emotions evolved across species to coordinate adaptive responses through behavioural control (Frijda, 1994b). If so, it becomes surprising that dominance, which precisely highlights individuals’ internal sense of behavioural influence over their surroundings, is considered peripheral to affect.
Finally, the valence-arousal model is a valuable tool within the JBT paradigm. It serves as an abstraction that helps to define input conditions (scenarios that precipitate a particular affective state), generate measurements of emotion’s effects (potential decisions in those scenarios), and probe causal dependencies between them (Sect. 2.2). However, it is essential to note that scientific models of affect can be regarded as relatively independent from theories of emotions rather than subordinate to them (for an overview of different degrees of independence, see Frigg & Hartmann, 2020). This means that core affect can be broken down into dimensions tailored to researchers’ aims, based on their relevance to the research question at hand, grounds of their plausibility, and ability to generate tractable hypotheses (Moors, 2022, p. 260). Therefore, to account for cases where wanting and liking do not align, we need to rethink our definition of core affect. In the next section, I propose a way to do this.
4 The VAD model in animal behaviour research
To develop a more ecologically oriented model of animal affect, let us begin by examining optimal foraging theory. This theory identifies two factors that influence animals’ decision-making process: “currency” and “constraints” (Pyke, 1984). Currency refers to the unit optimised by the animal, such as energy intake, while constraints are factors that limit foraging efficiency, such as predators’ presence. In other terms, currency is the measure by which foragers assess costs and benefits, while constraints relate to perceived behavioural limits. Similarly, I propose that a model of animal affective states should differentiate between valence (representing the weight that the organism attributes to fitness-enhancing or fitness-detrimental stimuli) and dominance (reflecting how prepared organisms perceive themselves to obtain the former and avoid the latter). However, questions arise about how these two dimensions, along with arousal, relate to neuronal systems, moods, and biases. I will address each of these questions in what follows.
4.1 Neuronal systems and discrete emotions
Decision-making requires considering available resources and constraints. To capture this idea, I propose that the Reward Acquisition System (RAS) guides organisms in obtaining positive and high-dominance states (cf. the green arrow in Fig. 4.1) and that the Punishment Avoidance System (PAS) operates to avoid negative, low-dominance states (cf. the orange arrow in Fig. 4.1). These systems come into play in situations where liking and wanting overlap. However, foraging animals can also encounter a broad spectrum of scenarios, ranging from highly rewarding yet dangerous stimuli to poorly rewarding yet safe surroundings. In these scenarios, it is assumed that organisms perform trade-offs to determine the best overall behavioural response. Nevertheless, evidence of sub-optimal behaviour suggests that their decision-making involves more than just determining trade-offs (Sect. 2.3).
Due to the relevance of efficiently navigating these complex scenarios, some species have evolved specific neural programs fine-tuned for this purpose. Following Panksepp (1998), we can assume that there are at least four such innate neural circuits in mammals: SEEKING, FEAR, RAGE, and PANIC. Among these, SEEKING prompts individuals to forage for rewards while dealing with punishing aspects of the world, while FEAR drives individuals to ensure survival (Wright & Panksepp, 2012). By mapping these emotional programs into the VAD model, we can better understand situations where liking and wanting do not overlap. In particular, SEEKING would maximise positive valence under challenging conditions (cf. the blue arrow in Fig. 4.2), while FEAR would assist individuals in securing safe, albeit less rewarding, outcomes (cf. the yellow arrow in Fig. 4.2).
VAD’s model space. In Fig. 4.1, the arrows indicate Mendl et al.’s (2010) putative biobehavioural systems, including the Reward Acquisition System (green) and the Punishment Avoidance System (orange). In contrast, Panksepp’s (1998) emotional programs SEEKING (blue) and FEAR (yellow) are featured in Fig. 4.2
Before moving further, two aspects of the model warrant attention. The first regards the grounds for incorporating discrete affective programs such as SEEKING and FEAR into the VAD model. Despite the standard view of discrete and dimensional models of affect as rivals, many authors have pointed out that there is psychological, neural, and behavioural evidence for both (Panksepp, 2007; Harmon-Jones et al., 2017; Mendl et al., 2010). Therefore, I assume that basic emotion programs and core affective dimensions co-exist in the brain, serving complementary functions. In this hybrid model, the VAD dimensions provide a functional scaffold for integrating basic affective programs such as SEEKING, FEAR, and other specialised neuronal circuits, enabling us to understand their interplay.
Secondly, it is important to clarify the differences between RAS and SEEKING. According to Wright and Panksepp (2012), reward circuits only involve hedonic pleasure, while SEEKING relates to foraging, action-oriented drives, correlating more with “euphoria” than pleasure. Following Bach and Dayan (2017), we can also appeal to instrumental reinforcement learning to elucidate this difference. In this framework, decision-making can be either “model-free” (when organisms select actions based on their value only) or “model-based” (when organisms additionally weigh the anticipated outcome of those actions). Therefore, since RAS only focus on the stimuli value—on how rewarding the stimulus is—it can be considered to trigger model-free decisions. In contrast, pre-programmed responses like SEEKING also consider anticipated outcomes—how those rewards are to be obtained—and thus can be considered to trigger model-based decisions.
4.2 Moods and decision-making
Moods are relatively enduring, objectless states that provide information about the prevailing environmental conditions the organism experiences (Mendl et al., 2010). For instance, a depressed individual may be inclined to perceive their environment as anticipating adverse outcomes (MacLeod & Byrne, 1996). In other terms, a depressed mood triggers decisions suited for dealing with those events; that is, it guides or “biases” cognitive responses. These moods may result from habitual exposure to punitive environments or innate behavioural dispositions.
Before specifying moods’ effects in the VAD model, an extensional definition of them must be provided. To characterise moods, I label each octant O1-O8 of the VAD space using Mehrabian’s (1996) model of personality traits (as depicted in Fig. 3). However, as observed in Sect. 3.1, states such as “Relaxed” or “Anxious” can be considered more appropriately belonging to the realm of moods, namely, occurrent object-less states. Personality traits will be discussed in Sect. 4.3.
Now, let us outline moods’ specific effects. In Mendl et al. (2010), different moods correlate to distinct attitudes. For instance, Q3 moods are associated with low expectations of positive events, while Q4 moods with a high expectation of adverse events (as illustrated in Fig. 1). Hence, alterations in arousal invert the expectation’s direction but not the state’s hedonic quality. When extrapolating this perspective into the VAD space, moods function analogously. That is, changes in arousal alter an individual’s expectations but not their state’s hedonic or dominant qualities. Hence, as Fig. 5 illustrates, we can classify moods into the following four groups:
-
The first class comprises the O1 mood (“Exuberant”; +V, +A, +D), which is associated with decision-making reflecting high expectations of safe and rewarding events, and the O2 mood (“Relaxed”; +V, –A, +D), which aligns with decisions indicating low expectations of non-rewarding, risky events.
-
The second class includes the O5 mood (“Dependent”; +V, +A, –D), which is associated with decisions reflecting the high expectation of rewarding yet risky events, and the O6 mood (“Docile”; +V, –A, –D), which indicates decisions reflecting the low expectation of non-rewarding yet safe events.
-
The third class consists of the O4 mood (“Hostile”; –V, +A, +D), correlated with decisions reflecting high expectations of non-rewarding but safe events, and the O3 mood (“Disdainful”; –V, –A, +D), which denotes decisions reflecting low expectations of rewarding but risky events.
-
Lastly, the fourth class includes the O8 (“Anxious”; –V, +A, –D), which corresponds to decisions reflecting the high expectation of non-rewarding, risky events, and the O7 (“Bored”; –V, –A, –D), which corresponds to decisions reflecting the low expectation of rewarding but safe events.
Before moving on, it is important to observe some differences between Mendl et al.’s (2010) model and our own. In the former, the mood marked by Q4 activates the PAS. In contrast, in the VAD model, we associate the activation of the PAS with the O8 mood (“Anxious”) and the activation of the FEAR circuit with the O4 mood (“Hostile”). Although anxiety may be considered to activate the FEAR program as well, it is worth acknowledging the practical role of the proposed affective labels in describing regions of the VAD space, regardless of how accurately they map our folk affective categories. As discussed in Sects. 5 and 6, the VAD model’s value lies in enabling us to develop new hypotheses about how different moods may bias decision-making (beyond the positive/negative dichotomy). Before that, we need to understand the role of such biases in the VAD model.
4.3 Cognitive biases
In Mendl et al. (2010), Q1 and Q2 moods are linked to positive expectations, while Q3 and Q4 are linked with negative expectations. The former moods are termed “optimist” and the latter “pessimist” biases in decision-making. In our model, in contrast, individuals who show enhanced expectation of (+ V, +D) events—those prone to an “Exuberant” or “Relaxed” mood—are optimists, and those that show enhanced expectations of (–V,–D) events—those inclined towards an “Anxious” or “Bored” mood—are classified as pessimist (see Fig. 6). Following Mendl et al. (2010), I assume that these biases hold particular relevance in situations where incoming sensory information is ambiguous, yet individuals’ survival depends on making an optimal decision.
As previously discussed, individual behavioural responses vary in terms of how organisms typically assess reward and safety trade-offs. Some organisms may consider that potential benefits outweigh the risks, while others may think otherwise. Mendl et al.’s (2010) model does not encompass these attitudes, which we can refer to as risk-prone and risk-averse attitudes. In the VAD model, we state that individuals who tend to favour (+ V,–D) events (that is, those who tend to be in a “Dependent” or “Docile” mood) are risk-prone, and conversely, those who tend to favour (–V, +D) events (that is, those who tend to be in a “Hostile” or “Disdainful” mood) are risk-averse. In Sect. 5, I argue that these states are particularly relevant in situations where incoming sensory information is ambivalent, yet the individual’s survival relies on making an optimal decision.
Let us analyse the relation between the cognitive biases. According to the VAD model, the optimism/pessimism and risk-prone/risk-averse continua are independent. As illustrated in Fig. 6, these attitudes constitute two orthogonal continua at 45 degrees of the valence and dominance dimensions. However, this assumption needs empirical investigation. For instance, we need to investigate whether organisms who tend to be more optimistic (with respect to ambiguous stimuli) also tend to display a higher inclination towards risk-prone behaviour (with respect to ambivalent stimuli). In Sect. 5, I discuss empirical evidence regarding this relationship.
Another aspect for consideration pertains to the relationship between these attitudes and personality traits, that is, stable behavioural responses. While optimist/pessimist or risk-prone/risk-averse biases can be indicative of a particular personality, they do not constitute sufficient evidence. In the field of biology, personality traits are considered to hinge not only on a singular type of observed behaviour but on various behaviours which the individual should display across a relatively wide timeframe (Kaiser & Müller, 2021). For instance, although individuals’ risk-prone attitudes may indicate that they possess a bold personality, it is necessary to complement this conclusion by examining the presence of additional boldness traits, such as the motivation to explore novel objects (Blaszczyk, 2017).
5 Applications (I): optimism vs. risk-taking
As we saw in Sect. 2.2, the JBT measures behavioural responses to experimental conditions possessing the potential for either reward or punishment, assumed to elicit positive or negative states, respectively. However, ecologically relevant situations present stimuli that intertwine both features. To understand animals’ decision-making within this kind of situation, I refer to the work of Bračić et al. (2023), a proof-of-principle experiment that highlights some of the limitations of the JBT. In this section, I argue that Bračić et al. study constitutes an example of how the VAD model can be effectively applied.
The authors highlight the need to test the potential ecological relevance of optimism and pessimism. To that effect, they introduce a novel testing protocol named the “Environment Choice Test” (ECT), designed to explore foraging decision-making under predation risk. Their study is composed of two experimental phases. The initial one involves the JBT, wherein laboratory mice are presented with ambiguous cues to assess their level of optimism, quantified as “optimism score”. In the second, the study incorporates the ECT, where mice are confronted with a choice between two chambers connected by short tunnels: (a) the “predator chamber”, laden with predator cues (e.g., illumination) but yielding a substantial reward and (b) “the safe chamber”, containing clean bedding yet yielding a small reward (see Fig. 7). The mice’s decision-making rates are quantified as “choice score”.Footnote 2
Environment Choice Test (Bračić et al., 2023). The test apparatus consists of five interconnected chambers. On each side, there are cue chambers where predator cues (“predator chamber”) or clean bedding (“safe chamber”) are introduced. During the test, mice need to traverse one of these chambers to finally reach a reward: by going through the predator chamber, they could claim a large reward and through a safe chamber, a small reward
The authors found that mice express consistent differences in their choices of the predator or safe chamber. However, they found no correlation between mice’s individual optimism and choice score, suggesting that optimists, although expecting positive outcomes, might not take more risks when it can jeopardise their survival. The authors discuss the possibility that these results may be due to the functioning of distinct or similar underlying cognitive mechanisms differently affected by previous life experiences.
As the ECT compels animals to strike a balance between avoiding predation exposure and capitalising on foraging opportunities, the VAD offers a framework for interpreting its results through the lens of affective states. To begin with, by juxtaposing conditions of small and large rewards with those of low and high risk, the predator and safe chambers can be respectively characterised as triggering (+ V, –D) and (–V, +D) states. Consequently, per the definition of attitudes outlined in Sect. 4.3, consistent reactions to these cues can reflect individuals’ attitudes: mice are risk-taking when favouring the predator chamber and risk-averse when evincing a predilection for the safe chamber. Aggregating these decisions can be alternatively referred to as a “risk score”.
Moreover, as we saw in Sect. 4.3, the VAD model posits that optimist/pessimist and risk-prone/risk-averse attitudes engage distinct behavioural systems. Specifically, the RAS/PAS and the SEEKING/FEAR systems, respectively (cf. Fig. 6). Thus, the model predicts the absence of a correlation between optimism and boldness moods. Bračić et al.’s finding that optimism and choice/risk scores do not correlate can be interpreted as substantiating such prediction, that is, as confirming the VAD model’s assumption that multiple attitudes may co-exist within the same individual (thus creating “behavioural syndromes”; see Sih et al., 2004).
The VAD model can also assist in further supporting research that involves probing the influence of an individual’s affective state on behavioural decision-making. In the JBT, it is studied to what extent individuals’ states of depression or anxiety influence their degree of optimism. Using the VAD, we could also investigate whether negative states influence their risk-taking propensity. In a new mechanistic study, one can, for example, induce stressors or other treatments on a randomly selected sub-group of individuals and then track whether and to what extent treatments make them risk-prone or risk-averse following Bračić et al.’s (2023) paradigm. Evidence showing that animals’ health (Heithaus et al., 2007), nutrition (Moran et al., 2021), and stress (Sih, 2011) affect their degree of risk-prone behaviour substantiate such hypothesis (however, see Niemelä & Dingemanse, 2018).
Moreover, we can go beyond valence-related priming. In an alternative type of manipulative experiment, one could employ negative priming that varies with respect to the degree of control/dominance. For example, we could use similar rewarding locations that present various degrees of safety or risk. In particular, equally poor food rewards could be placed in locations with more or less predator cues before testing. Subsequently, we would see how subgroups primed with a rewarding but non-dominant location vs. a dominant location thereby become more or less optimist or risk-prone. In this way, the VAD model provides a platform to test new hypotheses regarding mood-modulated decision-making through manipulative experiments.
It is essential to emphasise the advantage of conducting this type of behavioural study against the background of the VAD model. While the optimist-pessimist continuum has been functionally defined (using affective dimensions) as an individual’s expectations of positive or negative outcomes, the risk-taking continuum typically escaped such mapping. Thus, by unifying these two continua within a single multidimensional framework of affect, we can achieve a more complete picture of the structure of animals’ internal states (for insights into the unifying role of scientific explanations, see Kitcher, 1981).
6 Applications (II): hedonic vs. cognitive enrichments
Animal welfare science has traditionally focused on studying animals’ negative experiences, such as pain and stress. In recent years, however, the study of animals’ positive experiences has gained momentum, motivated by a desire to understand and promote “the conditions under which the animals thrive, not simply survive” (Hintze & Yee, 2023, p. 795).
It is challenging to describe and measure positive experiences accurately. In the field of psychology, happiness is thought to be achieved either by obtaining extrinsically rewarding outcomes or by pursuing an activity that is rewarding irrespective of any end-product, such as playing (Becker et al., 2009). Even though the pursuit of intrinsic rewards is believed to enhance well-being to a greater extent (Sheldon & Kasser, 1998), studies on animals targeting intrinsic motivation are relatively rare (see, however, Clark, 2023). In this section, I argue that the dominance dimension not only holds the potential to refine measurements and explanations regarding animal decision-making but also to provide insight into happiness-inducing living conditions.
A concept that drives the study of intrinsic motivation and well-being is “flow” (Csikszentmihalyi, 1990, 2002; Hintze & Yee, 2023; Clark, 2023)Footnote 3. Flow is a state of complete absorption in an autotelic activity that requires constructing a dynamic balance between an individual’s perceived skills and the degree of challenge the activity presents. To put it differently, flow requires that the task demands are maintained within a manageable scope without being predictive of the outcome. This balance is thus not static but unfolds throughout an activity by applying effort to a series of attainable goals, processing feedback regarding progress, and adapting action based on this feedback.
Crucially, satisfaction from an autotelic activity seems to be determined by a continuous equilibrium between the sensation of one’s capacity to temper with nature and the challenges it imposes. Thus, following the VAD model, I decompose affective states into their valence aspects, understood as subjective states mainly modulated by acquiring rewards such as food or shelter, and dominance aspects, understood as a subjective aspect modulated by the potential success in overcoming environmental challenges. Importantly, for both affective dimensions, long-term imbalances carry drawbacks. In humans, for example, an excessive abundance of resources has been observed to foster inertia, while scarcity of resources is correlated with depressive and anxiety disorders. Similarly, surmounting each challenge generates dullness, while failing to do so results in frustration (Csikszentmihalyi, 1990). Thus, drawing inspiration from Bakker et al. (2014), I illustrate the landscape of affective harmony as follows:
Harmony space. The levels of reward and control serve as indicators of harmony and disharmony in an animal’s perceived environment. The green and red dashed lines indicate hypothetical scenarios of an organism’s affective states fluctuating over time. The green line represents cycles characterised by successful competition for rewards, whereas the red lines represent cycles marked by unsuccessful competition
The grey area denotes the experience of harmony, while the area outside represents disharmony. Well-being hinges on a balanced interplay between excess or insufficiency of extrinsic rewards and between perceived skills and environmental challenges. In line with Mendl et al. (2010), this space allows us to chart an organism’s evolving affective trajectory within its environment, as driven by the moods it undergoes. In group foraging, for example, initially discovering a prey in an easily accessible location may elicit cycles of exuberance (+ V, +D) and then excitement (+ V,–D) due to the competition for this resource from other group members, as depicted by the green dashed line in Fig. 8. However, as depicted by the red dashed line, this cycle could be interrupted by another cycle where losing access to the shared reward may generate anxiety (–V,–D) or hostility (–V,–D). Insofar as green and red cycles do not become too intense or frequent, they can be said to constitute a harmonious experience.
Building upon this insight, we can improve the Environment Choice Test. As mentioned in Sect. 5, the ECT can include a phase where mice are randomly divided into two groups and exposed to either positive or negative enrichments. In an alternative version of this test, we can explore the potential for cognitive stimulation through flow induction instead of hedonic stimulation through the (di-)satisfaction of material necessities such as shelter or food (Clark, 2017). In particular, cognitive stimulation can be achieved by inducing animals to experience varying degrees of equilibrium and disequilibrium. For instance, researchers can move artificial prey on a rope to stimulate hunting and progressively adjust the challenge level by altering the speed or introducing unpredictable movements. To determine whether flow has been successfully induced, researchers can assess an animal’s resistance to increasingly attractive external distractions.Footnote 4
7 Conclusion
Animals possess a remarkable capacity to evaluate the quality of their environment. However, their behavioural responses are often at odds with what would be optimal to do. This paper has argued that this is because animals’ appraisal processes not only consider the perceived qualities of the environment—their valence—but also how capable they feel in dealing with them. By drawing from the literature on (self-)control in human psychology, this paper has proposed that, rather than peripheral, dominance is a significant aspect of core affect. Dominance stands as a theoretical tool that can unlock new avenues to understanding animal personality traits, moods, and emotions. By examining animals’ behavioural responses in terms of the interplay between valence and dominance, researchers can disentangle hedonic from motivational aspects and develop more ecologically valid experimental paradigms. Additionally, the proposed model has the potential to assist welfare scientists in developing new ideas about animals’ well-being by highlighting subjective phenomena that are often overlooked, namely, animals’ sense of control and, ultimately, their sense of agency.
Notes
Within the literature on agency, the subjective character of decision-making is termed “sense of control”. Pacherie (2007) delineates its three distinct components: (i) “rational control”, which involves ensuring the steps to attain a goal; (ii) “situational control”, which considers agents’ characteristics, the target of action and the surrounding context; and (iii) “motor control”, which specifies the detailed parameters of the selected motor program. The definition of dominance I propose aligns with situational control, albeit positioned as a subjective sensation on par with valence and arousal. Thanks to an anonymous reviewer for highlighting the necessity of exploring the relationship between the feeling of dominance and agency.
Note that, during the pre-training session, mice discovered that one side of the apparatus contains a large reward while other contains a small reward and learned how to reach them. When their accuracy reached 80%, they proceeded to the test.
Many thanks to Helene Richter for drawing my attention to the potential link between dominance and flow states.
While flow appears as an intriguing benchmark for understanding well-being, it is important to acknowledge that it represents a somewhat infrequent state of optimal experience. Not all experiences or situations aim to induce a flow state in animals. Hence, I introduce the concept of flow to show that animals’ sense of dominance can be helpful in characterising and potentially measuring episodes of optimal engagement and fulfilment (see Littlewood et al., 2023).
References
Asher, L., Friel, M., Griffin, K., & Collins, L. M. (2016). Mood and personality interact to determine cognitive biases in pigs. Biology Letters, 12(11), 20160402. https://doi.org/10.1098/rsbl.2016.0402
Bach, D. R., & Dayan, P. (2017). Algorithms for survival: A comparative perspective on emotions. Nature Reviews Neuroscience, 18(5), 311–319. https://doi.org/10.1038/nrn.2017.35
Bakker, I., Van Der Voordt, T., Vink, P., & De Boon, J. (2014). Pleasure, arousal, dominance: Mehrabian and Russell revisited. Current Psychology, 33, 405–421. https://doi.org/10.1007/s12144-014-9219-4
Barrett, L. F. (2017). How emotions are made: The secret life of the brain. Houghton-Mifflin-Harcourt.
Barrett, L. F., & Russell, J. A. (1999). The structure of current affect: Controversies and emerging consensus. Current Directions in Psychological Science, 8(1), 10–14. https://doi.org/10.1111/1467-8721.000
Becker, S., Bräscher, A. K., Bannister, S., Bensafi, M., Calma-Birling, D., Chan, R. C., Eerola, T., Ellingsen, D. M., Ferdenzi, C., Berridge, K. C., Robinson, T. E., & Aldridge, J. W. (2009). Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Current Opinion in Pharmacology, 9(1), 65–73. https://doi.org/10.1016/j.coph.2008.12.014
Blaszczyk, M. B. (2017). Boldness towards novel objects predicts predator inspection in wild vervet monkeys. Animal Behaviour, 123, 91–100. https://doi.org/10.1016/j.anbehav.2016.10.017
Bračić, M., Bierbaum, L., Peng, M., Nimalavachchlan, L., Siewert, V., Kaiser, S., Sachser, N., & Richter, H. (2023). The behavioural ecology of optimism: Exploring potential consequences of judgement bias in mice. Unpublished manuscript. Research Square. https://doi.org/10.21203/rs.3.rs-3071773/v1
Bryant, F. B. (1989). A four-factor model of perceived control: Avoiding, coping, obtaining, and savoring. Journal of Personality, 57(4), 773–797. https://doi.org/10.1111/j.1467-6494.1989.tb00494.x
Cabanac, M. (1992). Pleasure: The common currency. Journal of Theoretical Biology, 155(2), 173–200. https://doi.org/10.1016/S0022-5193(05)80594-6
Clark, F. E. (2017). Cognitive enrichment and welfare: Current approaches and future directions. Animal Behavior and Cognition, 4(1), 52–71. https://doi.org/10.12966/abc.05.02.2017
Clark, F. E. (2023). In the zone: Towards a comparative study of flow state in primates. Animal Behavior and Cognition, 10(1), 62–88. https://doi.org/10.26451/abc.10.01.04.2023
Csikszentmihalyi, M. (1990). Flow: The psychology of optimal experience. Harper and Row.
Csikszentmihalyi, M. (2002). Flow: The Classic Work on how to achieve happiness. Random House.
Dammhahn, M., & Almeling, L. (2012). Is risk-taking during foraging a personality trait? A field test for cross-context consistency in boldness. Animal Behaviour, 84(5), 1131–1139. https://doi.org/10.1016/j.anbehav.2012.08.014
De Waal, F. B., & Preston, S. D. (2017). Mammalian empathy: Behavioural manifestations and neural basis. Nature Reviews Neuroscience, 18(8), 498–509. https://doi.org/10.1038/nrn.2017.72
Deonna, J., & Teroni, F. (2012). The emotions: A philosophical introduction. Routledge.
Ekman, P. (1984). Expression and the nature of emotion. Approaches to Emotion, 3(19), 344.
Ellsworth, P. C., & Scherer, K. R. (2003). Appraisal processes in emotion. In R. J. Davidson, K. R. Scherer, & H. Hill Goldsmith (Eds.), Handbook of Affective sciences (pp. 572–595). Oxford University Press.
Frigg, R., & Hartmann, S. (2020). Models in Science. In Edward N. Zalta (Ed.), The Stanford Encyclopedia of Philosophy (Spring 2020 Edition). https://plato.stanford.edu/archives/spr2020/entries/models-science/
Frijda, N. H. (1994a). Varieties of affect: Emotions and episodes, moods, and sentiments. In P. Ekman, & R. J. Davidson (Eds.), The nature of emotion: Fundamental questions (pp. 59–67). Oxford University Press.
Frijda, N. (1994b). Emotions are functional, most of the time. In P. Ekman, & R. J. Davidson (Eds.), The nature of emotion: Fundamental questions (pp. 112–122). Oxford University Press.
Gregory, W. L. (1978). Locus of control for positive and negative outcomes. Journal of Personality and Social Psychology, 36(8), 840. https://doi.org/10.1037/0022-3514.36.8.840
Hansson, S. O. (2023). Risk. In Edward N. Zalta & Uri Nodelman (Eds.), The Stanford Encyclopedia of Philosophy (Summer 2023 Edition). https://plato.stanford.edu/archives/sum2023/entries/risk/
Harding, E. J., Paul, E. S., & Mendl, M. (2004). Cognitive bias and affective state. Nature, 427(6972), 312–312. https://doi.org/10.1038/427312a
Harmon-Jones, E., Harmon-Jones, C., & Summerell, E. (2017). On the importance of both dimensional and discrete models of emotion. Behavioral Sciences, 7(4), 66. https://doi.org/10.3390/bs7040066
Heithaus, M. R., Frid, A., Wirsing, A. J., Dill, L. M., Fourqurean, J. W., Burkholder, D., Thomson, J., & Bejder, L. (2007). State-dependent risk‐taking by green sea turtles mediates top‐down effects of tiger shark intimidation in a marine ecosystem. Journal of Animal Ecology, 76(5), 837–844. https://doi.org/10.1111/j.1365-2656.2007.01260.x
Hintze, S., & Yee, J. R. (2023). Animals in flow–towards the scientific study of intrinsic reward in animals. Biological Reviews, 98(3), 792–806. https://doi.org/10.1111/brv.12930
Kaiser, M. I., & Müller, C. (2021). What is an animal personality? Biology & Philosophy, 36(1). https://doi.org/10.1007/s10539-020-09776-w
Kenny, A. (1963). Action, emotion and will. Routledge and Kegan Paul; Humanities.
Kitcher, P. (1981). Explanatory unification. Philosophy of Science, 48(4), 507–531. https://doi.org/10.1007/s10539-020-09776-w
Lagisz, M., Zidar, J., Nakagawa, S., Neville, V., Sorato, E., Paul, E. S., Bateson, M., Mendl, M., & Løvlie, H. (2020). Optimism, pessimism and judgement bias in animals: A systematic review and meta-analysis. Neuroscience & Biobehavioral Reviews, 118, 3–17. https://doi.org/10.1016/j.neubiorev.2020.07.012
Lang, P. J. (1995). The emotion probe – studies of motivation and attention. American Psychologist, 50(5), 372–385. https://doi.org/10.1037/0003-066X.50.5.372
Laskowski, K. L., Chang, C. C., Sheehy, K., & Aguiñaga, J. (2022). Consistent individual behavioral variation: What do we know and where are we going? Annual Review of Ecology Evolution and Systematics, 53, 161–182. https://doi.org/10.1146/annurev-ecolsys-102220-011451
Littlewood, K. E., Heslop, M. V., & Cobb, M. L. (2023). The agency domain and behavioral interactions: Assessing positive animal welfare using the five domains model. Frontiers in Veterinary Science, 10, 1284869. https://doi.org/10.3389/fvets.2023.1284869
M Burghardt, G. (2019). A place for emotions in behavior systems research. Behavioural Processes, 166, 103881. https://doi.org/10.1016/j.beproc.2019.06.004
MacLeod, A. K., & Byrne, A. (1996). Anxiety, depression, and the anticipation of future positive and negative experiences. Journal of Abnormal Psychology, 105(2), 286.
Mehrabian, A. (1996). Pleasure-arousal-dominance: A general framework for describing and measuring individual differences in temperament. Current Psychology, 14, 261–292. https://doi.org/10.1007/BF02686918
Mehrabian, A., & Russell, J. A. (1974). An approach to environmental psychology. The MIT.
Mellor, D. J. (2016). Updating animal welfare thinking: Moving beyond the five freedoms towards a life worth living. Animals, 6, 21. https://doi.org/10.3390/ani6030021
Mendl, M., & Paul, E. S. (2020). Animal affect and decision-making. Neuroscience & Biobehavioral Reviews, 112, 144–163. https://doi.org/10.1016/j.neubiorev.2020.01.025
Mendl, M., Burman, O. H., & Paul, E. S. (2010). An integrative and functional framework for the study of animal emotion and mood. Proceedings of the Royal Society B: Biological Sciences, 277(1696), 2895–2904. https://doi.org/10.1098/rspb.2010.0303
Moors, A. (2022). Demystifying emotions: A typology of theories in psychology and philosophy. Cambridge University Press.
Moors, A., & De Houwer, J. (2005). Automatic processing of dominance and submissiveness. Experimental Psychology, 52(4), 296–302. https://doi.org/10.1027/1618-3169.52.4.296
Moran, N. P., Sánchez-Tójar, A., Schielzeth, H., & Reinhold, K. (2021). Poor nutritional condition promotes high‐risk behaviours: A systematic review and meta‐analysis. Biological Reviews, 96(1), 269–288. https://doi.org/10.1111/brv.12655
Neethirajan, S., Reimert, I., & Kemp, B. (2021). Measuring farm animal emotions—sensor-based approaches. Sensors 21(2):553 https://doi.org/10.3390/s21020553
Nematipour, B., Bračić, M., & Krohs, U. (2022). Cognitive bias in animal behavior science: A philosophical perspective. Animal Cognition, 25(4), 975–990. https://doi.org/10.1007/s10071-022-01647-z
Niemelä, P. T., & Dingemanse, N. J. (2018). Meta-analysis reveals weak associations between intrinsic state and personality. Proceedings of the Royal Society B: Biological Sciences, 285(1873), p20172823. https://doi.org/10.1098/rspb.2017.2823
Oosterhof, N. N., & Todorov, A. (2008). The functional basis of face evaluation. Proceedings of the National Academy of Sciences, 105(32), 11087–11092. https://doi.org/10.1073/pnas.0805664105
Osgood, C. E., Suci, G. J., & Tannenbaum, P. H. (1957). The measurement of meaning. University of Illinois Press.
Pacherie, E. (2007). The sense of control and the sense of agency. Psyche, 13(1), 1–30.
Panksepp, J. (1998). Affective neuroscience: The foundations of human and animal emotions. Oxford University Press.
Panksepp, J. (2007). Neurologizing the psychology of affects: How appraisal-based constructivism and basic emotion theory can coexist. Perspectives on Psychological Science, 2(3), 281–296. https://doi.org/10.1111/j.1745-6916.2007.00045.x
Paul, E. S., Harding, E. J., & Mendl, M. (2005). Measuring emotional processes in animals: The utility of a cognitive approach. Neuroscience & Biobehavioral Reviews, 29(3), 469–491. https://doi.org/10.1016/j.neubiorev.2005.01.002
Pyke, G. H. (1984). Optimal foraging theory: A critical review. Annual Review of Ecology and Systematics, 15(1), 523–575. https://doi.org/10.1146/annurev.es.15.110184.002515
Réale, D., Reader, S. M., Sol, D., McDougall, P. T., & Dingemanse, N. J. (2007). Integrating animal temperament within ecology and evolution. Biological Reviews, 82(2), 291–318. https://doi.org/10.1111/j.1469-185X.2007.00010.x
Rothbaum, F., Weisz, J. R., & Snyder, S. S. (1982). Changing the world and changing the self: A two-process model of perceived control. Journal of Personality and Social Psychology, 42(1), 5. https://doi.org/10.1037/0022-3514.42.1.5
Russell, J. A. (1978). Evidence of convergent validity on the dimensions of affect. Journal of Personality and Social Psychology, 36(10), 1152. https://doi.org/10.1037/0022-3514.36.10.1152
Russell, J. A. (1980). A circumplex model of affect. Journal of Personality and Social Psychology, 39(6), 1161. https://doi.org/10.1017/S0954579405050340
Russell, J. A., & Barrett, L. F. (1998). Core affect, prototypical emotional episodes, and other things called emotion: Dissecting the elephant. Journal of Personality and Social Psychology, 76(5), 805. https://doi.org/10.1037//0022-3514.76.5.805
Russell, J. A., & Mehrabian, A. (1977). Evidence for a three-factor theory of emotions. Journal of Research in Personality, 11(3), 273–294. https://doi.org/10.1016/0092-6566(77)90037-X
Scarantino, A., & de Sousa, R. (2018). Emotion. In Edward N. Zalta (Ed.), The Stanford Encyclopedia of Philosophy (Summer 2021 Edition). https://plato.stanford.edu/archives/sum2021/entries/emotion/
Scherer, K. R. (2009). The dynamic architecture of emotion: Evidence for the component process model. Cognition and Emotion, 23(7), 1307–1351. https://doi.org/10.1080/02699930902928969
Sheldon, K. M., & Kasser, T. (1998). Pursuing personal goals: Skills enable progress, but not all progress is beneficial. Personality and Social Psychology Bulletin, 24(12), 1319–1331. https://doi.org/10.1177/01461672982412006
Sih, A. (2011). Effects of early stress on behavioral syndromes: An integrated adaptive perspective. Neuroscience & Biobehavioral Reviews, 35(7), 1452–1465. https://doi.org/10.1016/j.neubiorev.2011.03.015
Sih, A., Bell, A., & Johnson, J. C. (2004). Behavioral syndromes: An ecological and evolutionary overview. Trends in Ecology & Evolution, 19(7), 372–378. https://doi.org/10.1016/j.tree.2004.04.009
Verdolin, J. L. (2006). Meta-analysis of foraging and predation risk trade-offs in terrestrial systems. Behavioral Ecology and Sociobiology, 60, 457–464. https://doi.org/10.1007/s00265-006-0172-6
Watson, D., Wiese, D., Vaidya, J., & Tellegen, A. (1999). The two general activation systems of affect: Structural findings, evolutionary considerations, and psychobiological evidence. Journal of Personality and Social Psychology, 76(5), 820. https://doi.org/10.1037/0022-3514.76.5.820
Webber, S., Cobb, M., & Coe, J. C. (2022). June 30). Welfare through competence: A framework for animal-centric technology design. Frontiers in Veterinary Science, 9, 885973. https://doi.org/10.3389/fvets.2022.885973
Wright, J. S., & Panksepp, J. (2012). An evolutionary framework to understand foraging, wanting, and desire: The neuropsychology of the SEEKING system. Neuropsychoanalysis, 14(1), 5–39. https://doi.org/10.1080/15294145.2012.10773683
Acknowledgements
I want to express my gratitude to Ulrich Krohs, Helene Richter, Melanie Dammhahn, Lea Mascherrek, Jochen Müller, Behzad Nematipour, and Marko Bračić, as well as to the participants of the NC3 Seminar at the University of Münster and the Inaugural Conference of the Philosophy of Animal Minds and Behavior Association (PAMBA) for their feedback on early versions of this paper. Any remaining errors or omissions are mine. This research was funded by the German Research Foundation (DFG) as part of the CRC TRR 212 (NC3) – Project number 316099922.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that there are no conflicts of interest to disclose in relation to this research.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Carranza-Pinedo, V. Rethinking core affect: the role of dominance in animal behaviour and welfare research. Synthese 203, 153 (2024). https://doi.org/10.1007/s11229-024-04591-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11229-024-04591-2