Abstract
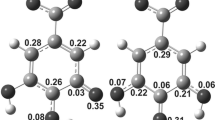
To understand the scavenging action of chlorophyll (Chla) found in most of the vegetables towards hydroxyl (\({{\text{OH}}}^{\bullet }\)) radicals, its reactions with \({{\text{OH}}}^{\bullet }\) radicals via RAF, HAT, and SET mechanisms have been investigated theoretically using two layer ONIOM [M06-2X/6-31G(d) (High):M06-2X/3-21G (Low)] method and M06-2X/6–311 + G(d,p) level of density functional theory. The molecular electrostatic potential (MEP), highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) plots, HOMO–LUMO energy gap, global hardness (η), global softness (S), electronegativity (χ), and electrophilicity index (ω) of Chla molecule were computed and analyzed to determine its stability and reactive sites. It is found that RAF and HAT reactions are exergonic in both gaseous and aqueous media whereas SET reactions are endergonic in both media. However, all the RAF, HAT and SET reactions studied here are found to be more favourable in aqueous media vs. gas phase. The rate constants of RAF reactions at different sites are found to be of the order of ~ 6.2 × 107–1.8 × 1010 s−1 indicating that RAF reactions would be appreciably fast. This study concludes that chlorophyll can efficiently scavenge \({{\text{OH}}}^{\bullet }\) radicals preferably via RAF and HAT mechanisms and intake of water with chlorophyll can enhance its scavenging actions.









Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article.
References
Phaniendra A, Jestadi DB, Periyasamy L (2015) Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem 30(1):11–26
YlÄ-Herttuala S (1999) Oxidized LDL and Atherogenesis a. Ann N Y Acad Sci 874(1):134–137
Stadtman ER, Levine RL (2000) Protein oxidation. Ann N Y Acad Sci 899(1):191–208
Marnett LJ (2000) Oxyradicals and DNA damage. Carcinogenesis 21(3):361–370
Harman D (1988) Free radicals in aging. Mol Cell Biochem 84(2):155–161
Jena N (2012) DNA damage by reactive species: mechanisms, mutation and repair. J Biosci 37(3):503–517
Siriwardhana N, Shahidi F, Jeon YJ (2006) Potential antioxidative effects of cactus pear fruit (Opuntia ficus-indica) extract on radical scavenging and DNA damage reduction in human peripheral lymphocytes. J Food Lipids 13(4):445–458
Bedwell S, Dean R, Jessup W (1989) The action of defined oxygen-centred free radicals on human low-density lipoprotein. Biochem J 262(3):707–712
Halliwell B (1987) Oxidants and human disease: some new concepts 1. FASEB J 1(5):358–364
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84
Pham-Huy LA, He H, Pham-Huy C (2008) Free radicals, antioxidants in disease and health. Int J Biomed Sci: IJBS 4(2):89
Cecarini V, Gee J, Fioretti E, Amici M, Angeletti M, Eleuteri AM, Keller JN (2007) Protein oxidation and cellular homeostasis: emphasis on metabolism. Biochim Biophys Acta (BBA)-Mol Cell Res 1773(2):93–104
Carocho M, Ferreira IC (2013) A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol 51:15–25
Rahman K (2007) Studies on free radicals, antioxidants, and co-factors. Clin Interv Aging 2(2):219
Block G, Patterson B, Subar A (1992) Fruit, vegetables, and cancer prevention: a review of the epidemiological evidence. Nutr Cancer 18(1):1–29
Lanfer-Marquez UM, Barros RM, Sinnecker P (2005) Antioxidant activity of chlorophylls and their derivatives. Food Res Int 38(8–9):885–891
Kephart JC (1955) Chlorophyll derivatives—Their chemistry? commercial preparation and uses. Econ Bot 9(1):3–38
Edwards B (1954) Treatment of chronic leg ulcers with ointment containing soluble chlorophyll. Physiotherapy 40(6):177–179
Larato D, Pfau F (1970) Effects of a water-soluble chlorophyllin ointment on gingival inflammation. N Y State Dent J 36(5):291–293
Young RW, Beregi JS Jr (1980) Use of chlorophyllin in the care of geriatric patients. J Am Geriatr Soc 28(1):46–47
Lai C-N, Butler MA, Matney TS (1980) Antimutagenic activities of common vegetables and their chlorophyll content. Mutat Res/Genet Toxicol 77(3):245–250
Ferruzzi MG, Blakeslee J (2007) Digestion, absorption, and cancer preventative activity of dietary chlorophyll derivatives. Nutr Res 27(1):1–12
Vázquez-Durán A, Téllez-Isaías G, Hernández-Rodríguez M, Ruvalcaba RM, Martínez J, Nicolás-Vázquez MI, Aceves-Hernández JM, Méndez-Albores A (2022) The ability of chlorophyll to trap carcinogen aflatoxin B1: a theoretical approach. Int J Mol Sci 23(11):6068
Ferruzzi M, Böhm V, Courtney P, Schwartz S (2002) Antioxidant and antimutagenic activity of dietary chlorophyll derivatives determined by radical scavenging and bacterial reverse mutagenesis assays. J Food Sci 67(7):2589–2595
Hoshina C, Tomita K, Shioi Y (1998) Antioxidant activity of chlorophylls: its structure-activity relationship. In: Garab G (ed) Photosynthesis: Mechanisms and Effects. Springer, Dordrecht. https://doi.org/10.1007/978-94-011-3953-3_766
Hsu C-Y, Chao P-Y, Hu S-P, Yang C-M (2013) The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr 4:1–8
Dey G, Naik D, Moorthy P (1999) Pulse radiolysis studies on redox reactions of gallic acid: one electron oxidation of gallic acid by gallic acid–OH adduct. Phys Chem Chem Phys 1(8):1915–1918
Marino T, Galano A, Russo N (2014) Radical scavenging ability of gallic acid toward OH and OOH radicals. Reaction mechanism and rate constants from the density functional theory. J Phys Chem B 118(35):10380–10389
Castañeda-Arriaga R, Perez-Gonzalez A, Marino T, Russo N, Galano A (2021) Antioxidants into nopal (opuntia ficus-indica), important inhibitors of free radicals’ formation. Antioxidants 10(12):2006
Leopoldini M, Chiodo SG, Russo N, Toscano M (2011) Detailed investigation of the OH radical quenching by natural antioxidant caffeic acid studied by quantum mechanical models. J Chem Theory Comput 7(12):4218–4233
Loussouarn M, Krieger-Liszkay A, Svilar L, Bily A, Birtić S, Havaux M (2017) Carnosic acid and carnosol, two major antioxidants of rosemary, act through different mechanisms. Plant Physiol 175(3):1381–1394
Hernandez DA, Tenorio FJ (2018) Reactivity indexes of antioxidant molecules from Rosmarinus officinalis. Struct Chem 29(3):741–751
Husain SR, Cillard J, Cillard P (1987) Hydroxyl radical scavenging activity of flavonoids. Phytochemistry 26(9):2489–2491
Agnihotri N, Mishra P (2011) Scavenging mechanism of curcumin toward the hydroxyl radical: a theoretical study of reactions producing ferulic acid and vanillin. J Phys Chem A 115(49):14221–14232
Dhaouadi Z, Nsangou M, Garrab N, Anouar E, Marakchi K, Lahmar S (2009) DFT study of the reaction of quercetin with· O2-and· OH radicals. J Mol Struct (Thoechem) 904(1–3):35–42
Anderson RF, Fisher LJ, Hara Y, Harris T, Mak WB, Melton LD, Packer JE (2001) Green tea catechins partially protect DNA from· OH radical-induced strand breaks and base damage through fast chemical repair of DNA radicals. Carcinogenesis 22(8):1189–1193
Mahmoudi S, Dehkordi MM, Asgarshamsi MH (2021) Density functional theory studies of the antioxidants—a review. J Mol Model 27(9):271
Chung LW, Sameera W, Ramozzi R, Page AJ, Hatanaka M, Petrova GP, Harris TV, Li X, Ke Z, Liu F, Li HB (2015) The ONIOM method and its applications. Chem Rev 115(12):5678–5796
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theoret Chem Acc 120(1–3):215–241
Bechaieb R, Lakhdar ZB, Gérard H (2018) DFT and TD-DFT studies of Mg-substitution in chlorophyll by Cr (II), Fe (II) and Ni (II). Chem Afr 1(1):79–86
Sinnecker S, Koch W, Lubitz W (2002) Chlorophyll a radical ions: a density functional study. J Phys Chem B 106(20):5281–5288
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65(2):239–245
Skodje RT, Truhlar DG (1981) Parabolic tunneling calculations. J Phys Chem 85(6):624–628
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakattsuji H, Caricato M, Li X, Hratchian HP, Lzmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09 C. 01. Gaussian, Inc., Wallingford CT
Dennington R, Keith T, Millam J (2009) GaussView, version 5
Fukui K, Yonezawa T, Nagata C, Shingu H (1954) Molecular orbital theory of orientation in aromatic, heteroaromatic, and other conjugated molecules. J Chem Phys 22(8):1433–1442
Parcheta M, Świsłocka R, Orzechowska S, Akimowicz M, Choińska R, Lewandowski W (2021) Recent developments in effective antioxidants: the structure and antioxidant properties. Materials 14(8):1984
Huq F (2007) Molecular modelling analysis of the metabolism of fentanyl. J Pharmacol Toxicol 2(2):176–182
Acknowledgements
Authors would like to acknowledge the general computational facility of the Department of Physics, Assam University, Silchar, India. Authors would like to thank Dr. María Inés Nicolás-Vázquez for providing coordinate of the chlorophyll molecule for the present study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
S.B. planned, performed simulation, collected data and contributed partly in writing. P.K.S. planned and supervised the research work, analysis of the results and writing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
This article does not contain any studies involving animals performed by any of the authors.
Consent to participate
This article does not contain any studies involving animals performed by any of the authors.
Consent to publish
All the authors mentioned in the manuscript have given consent for submission and subsequent publication of the manuscript.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
11224_2024_2331_MOESM1_ESM.docx
The optimized structures of adducts formed in RAF reactions of Chla and Mchla; Table containing ESP charges of Chla and MChla; reaction free energy and corresponding Boltzmann Populations for Chla and MChla; imaginary frequencies of TSs and tunneling constants; rate constant for the study of RAF involving MChla model; reaction free energies involved in SET mechanism, spin density located at HAT reaction sites, and the Cartesian coordinates of all the optimized TSs involved in RAF for MChla model are provided as Supporting Information. The online version containing supplementary material available at…… (DOCX 10442 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Biswas, S., Shukla, P.K. Reactions of chlorophyll with hydroxyl radicals via RAF, HAT and SET mechanisms: A theoretical study. Struct Chem (2024). https://doi.org/10.1007/s11224-024-02331-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-024-02331-3