Abstract
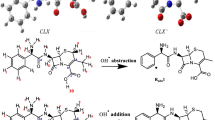
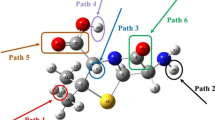
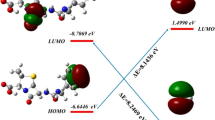
The presence of pharmaceuticals such as the antibiotic cephalexin in aqueous environments increases public health concerns due to their adverse biological effects and antibiotic resistance. It may be promising to remove these compounds from the aquatic environment through degradation reactions that convert them into non-toxic products. For this purpose, Density Functional Theory (DFT) molecular orbital calculations were performed to investigate the kinetics and mechanism of the degradation reaction of cephalexin with the hydroxyl (OH) radical. Reaction rate constants and branching ratios for 11 different reaction paths were calculated in the temperature range of 200 to 400 K. The total rate constant was calculated as 7.05 × 109 M−1 s−1 and is in good agreement with the experimental value. According to the kinetic and thermodynamic results, it can be concluded that the hydroxyl radical preferentially attacks the beta-lactam ring. The effect of water on the reaction mechanism was investigated in both implicit and explicit solvation models. Explicitly added water molecules affect the degradation reaction kinetic so that the results become compatible with the experimental ones. Ecotoxicity and bioaccumulation calculations on cephalexin and its degradation products show that some of its degradation products are harmful.








Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Arsand JB, Hoff RB, Jank L et al (2018) Transformation products of amoxicillin and ampicillin after photolysis in aqueous matrices: identification and kinetics. Sci Total Environ 642:954–967. https://doi.org/10.1016/j.scitotenv.2018.06.122
Karlesa A, De Vera GAD, Dodd MC et al (2014) Ferrate(VI) oxidation of β-lactam antibiotics: reaction kinetics, antibacterial activity changes, and transformation products. Environ Sci Technol 48:10380–10389. https://doi.org/10.1021/es5028426
Szabó L, Tóth T, Rácz G et al (2016) •OH and e-aq are yet good candidates for demolishing the β-lactam system of a penicillin eliminating the antimicrobial activity. Radiat Phys Chem 124:84–90. https://doi.org/10.1016/j.radphyschem.2015.10.012
Serna-Galvis EA, Giraldo-Aguirre AL, Silva-Agredo J et al (2017) Removal of antibiotic cloxacillin by means of electrochemical oxidation, TiO2 photocatalysis, and photo-Fenton processes: analysis of degradation pathways and effect of the water matrix on the elimination of antimicrobial activity. Environ Sci Pollut Res 24:6339–6352. https://doi.org/10.1007/s11356-016-6257-5
He J, Zhang Y, Guo Y et al (2019) Photocatalytic degradation of cephalexin by ZnO nanowires under simulated sunlight: kinetics, influencing factors, and mechanisms. Environ Int. https://doi.org/10.1016/j.envint.2019.105105
Giraldo-Aguirre AL, Serna-Galvis EA, Erazo-Erazo ED et al (2018) Removal of β-lactam antibiotics from pharmaceutical wastewaters using photo-Fenton process at near-neutral pH. Environ Sci Pollut Res 25:20293–20303. https://doi.org/10.1007/s11356-017-8420-z
Qian Y, Liu X, Li K et al (2020) Enhanced degradation of cephalosporin antibiotics by matrix components during thermally activated persulfate oxidation process. Chem Eng J. https://doi.org/10.1016/j.cej.2019.123332
Serna-Galvis EA, Silva-Agredo J, Giraldo AL et al (2016) Comparison of route, mechanism and extent of treatment for the degradation of a β-lactam antibiotic by TiO2 photocatalysis, sonochemistry, electrochemistry and the photo-Fenton system. Chem Eng J 284:953–962. https://doi.org/10.1016/j.cej.2015.08.154
Ajoudanian N, Nezamzadeh-Ejhieh A (2015) Enhanced photocatalytic activity of nickel oxide supported on clinoptilolite nanoparticles for the photodegradation of aqueous cephalexin. Mater Sci Semicond Process 36:162–169. https://doi.org/10.1016/j.mssp.2015.03.042
Masmoudi R, Khettaf S, Soltani A et al (2022) Cephalexin degradation initiated by OH radicals: theoretical prediction of the mechanisms and the toxicity of byproducts. J Mol Model. https://doi.org/10.1007/s00894-022-05121-y
Sun X, He W, Yang T et al (2021) Ternary TiO2/WO3/CQDs nanocomposites for enhanced photocatalytic mineralization of aqueous cephalexin: degradation mechanism and toxicity evaluation. Chem Eng J. https://doi.org/10.1016/j.cej.2021.128679
Ribeiro AR, Sures B, Schmidt TC (2018) Cephalosporin antibiotics in the aquatic environment: a critical review of occurrence, fate, ecotoxicity and removal technologies. Environ Pollut 241:1153–1166
Chen Y, Lv G, Wang Y et al (2023) The oxidation mechanism and kinetics of limononic acid by hydroxyl radical in atmospheric aqueous phase. Atmos Environ. https://doi.org/10.1016/j.atmosenv.2022.119527
Song W, Chen W, Cooper WJ et al (2008) Free-radical destruction of β-lactam antibiotics in aqueous solution. J Phys Chem A 112:7411–7417. https://doi.org/10.1021/jp803229a
Li N, Tian Y, Zhao J et al (2018) Z-scheme 2D/3D g-C3N4@ZnO with enhanced photocatalytic activity for cephalexin oxidation under solar light. Chem Eng J 352:412–422. https://doi.org/10.1016/j.cej.2018.07.038
Antonin VS, Aquino JM, Silva BF et al (2019) Comparative study on the degradation of cephalexin by four electrochemical advanced oxidation processes: evolution of oxidation intermediates and antimicrobial activity. Chem Eng J 372:1104–1112. https://doi.org/10.1016/j.cej.2019.04.185
Al-Musawi TJ, Kamani H, Bazrafshan E et al (2019) Optimization the effects of physicochemical parameters on the degradation of cephalexin in sono-Fenton reactor by using Box-Behnken response surface methodology. Catal Letters 149:1186–1196. https://doi.org/10.1007/s10562-019-02713-x
Bansal P, Verma A (2017) Synergistic effect of dual process (photocatalysis and photo-Fenton) for the degradation of cephalexin using TiO2 immobilized novel clay beads with waste fly ash/foundry sand. J Photochem Photobiol A Chem 342:131–142. https://doi.org/10.1016/j.jphotochem.2017.04.010
Coledam DAC, Pupo MMS, Silva BF et al (2017) Electrochemical mineralization of cephalexin using a conductive diamond anode: a mechanistic and toxicity investigation. Chemosphere 168:638–647. https://doi.org/10.1016/j.chemosphere.2016.11.013
Gashtasbi F, Yengejeh RJ, Babaei AA (2018) Photocatalysis assisted by activated-carbon-impregnated magnetite composite for removal of cephalexin from aqueous solution. Korean J Chem Eng 35:1726–1734. https://doi.org/10.1007/s11814-018-0061-5
Darwish M, Mohammadi A, Assi N (2016) Integration of nickel doping with loading on graphene for enhanced adsorptive and catalytic properties of CdS nanoparticles towards visible light degradation of some antibiotics. J Hazard Mater 320:304–314. https://doi.org/10.1016/j.jhazmat.2016.08.043
Gao Y, Ji Y, Li G, An T (2014) Mechanism, kinetics and toxicity assessment of OH-initiated transformation of triclosan in aquatic environments. Water Res 49:360–370. https://doi.org/10.1016/j.watres.2013.10.027
Ghasemi M, Khataee A, Gholami P et al (2020) In-situ electro-generation and activation of hydrogen peroxide using a CuFeNLDH-CNTs modified graphite cathode for degradation of cefazolin. J Environ Manag. https://doi.org/10.1016/j.jenvman.2020.110629
Aydogdu S, Hatipoglu A (2023) Theoretical insights into the reaction mechanism and kinetics of ampicillin degradation with hydroxyl radical. J Mol Model. https://doi.org/10.1007/s00894-023-05462-2
Ngo TC, Taamalli S, Srour Z et al (2023) Theoretical insights into the oxidation of quinmerac herbicide initiated by HO• radical in aqueous media: mechanism, kinetics, and ecotoxicity. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2023.109941
Aydogdu S, Hatipoglu A, Eren B, Gurkan YY (2021) Photodegradation kinetics of organophosphorous with hydroxyl radicals: experimental and theoretical study. J Serb Chem Soc 86:955–969. https://doi.org/10.2298/JSC210409056A
Yang B, Liang A, Wang L (2023) The atmospheric oxidation mechanism of acetophenone initiated by the hydroxyl radicals. Atmos Environ. https://doi.org/10.1016/j.atmosenv.2023.119905
Yao J, Tang Y, Zhang Y, Ruan M, Wu W, Sun J (2022) New theoretical investigation of mechanism, kinetics, and toxicity in the degradation of dimetridazole and ornidazole by hydroxyl radicals in aqueous phase. J Hazard Mater 422:126930. https://doi.org/10.1016/j.jhazmat.2021.126930
Sun J, Chu R, Khan ZUH (2023) A theoretical study on the degradation mechanism, kinetics, and ecotoxicity of metronidazole (MNZ) in •OH- and SO4•−-assisted advanced oxidation processes. Toxics 11:796. https://doi.org/10.3390/toxics11090796
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams-Young D, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery JA, Peralta Jr. JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16, Revision C.01, Gaussian, Inc., Wallingford CT
Dong S, Bi S (2019) The solvation effect on the rattling behaviour of the hydrated excess proton in water. Phys Chem Chem Phys 21:22385–22389. https://doi.org/10.1039/c9cp03827b
Aydogdu S, Hatipoglu A (2023) Aqueous degradation of 6-APA by hydroxyl radical: a theoretical study. J Mol Model. https://doi.org/10.1007/s00894-023-05636-y
Hosseini SJ (2023) Solvent effects on direct and indirect tautomerism of pyrimidin-2(1H)-one/pyrimidin-2-ol. J Phys Org Chem. https://doi.org/10.1002/poc.4473
Foresman J, Frish E (1996) Exploring Chemistry with Electronic Structure Methods. Gaussian Inc, Pittsburg
Dennington RD, Keith TA, Millam JM (2008) GaussView 5.0. 9. Gaussian Inc, Wallingford
Levine IN (2009) Physical Chemistry, 6th edn. McGraw Hill-Higher Education, New York
El-hadj Saїd A, Mekelleche SM (2021) Antioxidant activity of Trolox derivatives toward methylperoxyl radicals: thermodynamic and kinetic theoretical study. Theor Chem Acc. https://doi.org/10.1007/s00214-021-02815-z
Collins FC, Kimball GE (1949) Diffusion-controlled reaction rates. J Colloid Sci 4:425–454
ECOSAR (2023) Ecological Structure Activity Relationships (ECOSAR) Predictive Model. https://www.epa.gov/tsca-screening-tools/ecological-structure-activity-relationships-ecosar-predictive-model
T.E.S.T (2020) Toxicity estimation software tool, Washington DC. https://www.epa.gov/chemical-research/toxicity-estimation-software-tool-test
EPISuite™ (2023) Estimation program interface, Washington DC. https://www.epa.gov/tsca-screening-tools/epi-suitetm-estimation-program-interface
Serna-Galvis EA, Ferraro F, Silva-Agredo J, Torres-Palma RA (2017) Degradation of highly consumed fluoroquinolones, penicillins and cephalosporins in distilled water and simulated hospital wastewater by UV254 and UV254/persulfate processes. Water Res 122:128–138. https://doi.org/10.1016/j.watres.2017.05.065
Li H, Zhang Y, Wan J et al (2018) Theoretical investigation on the oxidation mechanism of dibutyl phthalate by hydroxyl and sulfate radicals in the gas and aqueous phase. Chem Eng J 339:381–392. https://doi.org/10.1016/j.cej.2017.12.153
Li S, Huang T, Du P et al (2020) Photocatalytic transformation fate and toxicity of ciprofloxacin related to dissociation species: experimental and theoretical evidences. Water Res. https://doi.org/10.1016/j.watres.2020.116286
Uc VH, Alvarez-Idaboy JR, Galano A et al (2006) Theoretical determination of the rate constant for OH hydrogen abstraction from toluene. J Phys Chem A 110:10155–10162. https://doi.org/10.1021/jp062775l
Dao DQ, Taamalli S, Louis F et al (2023) Hydroxyl radical-initiated decomposition of metazachlor herbicide in the gaseous and aqueous phases: mechanism, kinetics, and toxicity evaluation. Chemosphere. https://doi.org/10.1016/j.chemosphere.2022.137234
Vione D, De Laurentiis E, Berto S et al (2016) Modeling the photochemical transformation of nitrobenzene under conditions relevant to sunlit surface waters: reaction pathways and formation of intermediates. Chemosphere 145:277–283. https://doi.org/10.1016/j.chemosphere.2015.11.039
Eberhardt MK, Yoshida M (1973) Radiation-induced homolytic aromatic substitution. I. Hydroxylation of nitrobenzene, chlorobenzene, and toluene. J Phys Chem 77:589–597. https://doi.org/10.1021/j100624a005
Homem V, Santos L (2011) Degradation and removal methods of antibiotics from aqueous matrices - a review. J Environ Manag 92:2304–2347
Serna-Galvis EA, Montoya-Rodríguez D, Isaza-Pineda L et al (2019) Sonochemical degradation of antibiotics from representative classes-considerations on structural effects, initial transformation products, antimicrobial activity and matrix. Ultrason Sonochem 50:157–165. https://doi.org/10.1016/j.ultsonch.2018.09.012
Wang XH, Lin AYC (2012) Phototransformation of cephalosporin antibiotics in an aqueous environment results in higher toxicity. Environ Sci Technol 46:12417–12426. https://doi.org/10.1021/es301929e
Dodd MC, Buffle MO, Von Gunten U (2006) Oxidation of antibacterial molecules by aqueous ozone: moiety-specific reaction kinetics and application to ozone-based wastewater treatment. Environ Sci Technol 40:1969–1977. https://doi.org/10.1021/es051369x
Wojnárovits L, Tóth T, Takács E (2018) Critical evaluation of rate coefficients for hydroxyl radical reactions with antibiotics: a review. Crit Rev Environ Sci Technol 48:575–613. https://doi.org/10.1080/10643389.2018.1463066
Klasmeier J, Matthies M, Macleod M et al (2006) Application of multimedia models for screening assessment of long-range transport potential and overall persistence. Environ Sci Technol 40:53–60. https://doi.org/10.1021/es0512024
Barone V, Cossi M, Tomasi J (1998) Geometry optimization of molecular structures in solution by the polarizable continuum model. J Phys Chem A 102:1995–2001
Klamt A, Moya C, Palomar J (2015) A comprehensive comparison of the IEFPCM and SS(V)PE continuum solvation methods with the COSMO approach. J Chem Theory Comput 11:4220–4225. https://doi.org/10.1021/acs.jctc.5b00601
Barone V, Cossi M (1998) Quantum calculation of molecular energies and energy gradients in solution by a conductor solvent model. J Phys Chem A 102:1995–2001. https://doi.org/10.1021/jp9716997
Gonzalez MG, Oliveros E, Wörner M, Braun AM (2004) Vacuum-ultraviolet photolysis of aqueous reaction systems. J Photochem Photobiol, C 5:225–246
Gonzalez C, Schlegel HB (1990) Reaction path following in mass-weighted internal coordinates. J Phys Chem 94:5523–5527
Boyd AA, Flaud PM, Daugey N, Lesclaux R (2003) Rate constants for RO2 + HO2 reactions measured under a large excess of HO2. J Phys Chem A 107:818–821. https://doi.org/10.1021/jp026581r
Hammond GS (1955) A correlation of reaction rates. J Am Chem Soc 77:334–338. https://doi.org/10.1021/ja01607a027
Zhang Y, Wei B, Tang R (2023) Theoretical study on the mechanisms, kinetics, and toxicity evaluation of OH-initiated atmospheric oxidation reactions of coniferyl alcohol. Atmosphere (Basel). https://doi.org/10.3390/atmos14060976
Conder JM, Hoke RA, De Wolf W et al (2008) Are PFCAs bioaccumulative? A critical review and comparison with regulatory criteria and persistent lipophilic compounds. Environ Sci Technol 42:995–1003
Funding
This study is supported by Yildiz Technical University Research Coordination with Project number FDK-2021–4138. We also acknowledge that computational resources are provided by TÜBİTAK ULAKBİM, High Performance and Grid Computing Center (TRUBA).
Author information
Authors and Affiliations
Contributions
Arzu Hatipoglu: conceptualization and writing—reviewing and editing. Seyda Aydogdu: DFT calculations and writing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aydogdu, S., Hatipoglu, A. Degradation kinetics and prediction of primary intermediates of cephalexin in aqueous media. Struct Chem (2024). https://doi.org/10.1007/s11224-024-02311-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-024-02311-7