Abstract
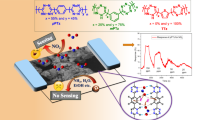
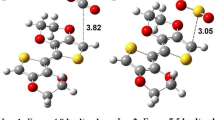
The investigation into conducting organic polymers and cyanogen halides (COPs-CNX) complexes’ optimization at the M06-2X/6-31+G(d) level, alongside subsequent analyses, provides valuable insights into the interaction mechanisms between COPs and CNX molecules. The basis set superposition error (BSSE)–corrected interaction energies emphasize the pronounced strength of electrostatic interaction within polyaniline and cyanogen halides (PANI ES-CNX) complexes. Conversely, poly(3,4-ethylenedioxythiophene) (PEDOT), polypyrrole (PPy), and polythiophene (PTh) exhibit weak interactions with CNX. Natural bond orbital (NBO) analysis confirms strong interactions between CNX and PANI ES through nitrogen sites, while halogen sites mediate interactions between CNX and PEDOT, PPy, and PTh. Charge transfer analysis underscores increased transfer within PANI ES-CNX complexes, indicating a stronger interaction. Topological analysis reveals non-covalent electrostatic interactions between COPs and CNX, notably stronger in PANI ES-CNX complexes. The outcomes position PANI ES as a promising candidate for CNX sensing, particularly in CNBr detection. Furthermore, this study computes the recovery time for COPs-CNX complexes, further contributing to the understanding of their applicability in sensing applications.




Similar content being viewed by others
Data availability
No datasets were generated or analysed during the current study.
References
Kononen DW (1988) Acute toxicity of cyanogen chloride to Daphnia magna. Bull Environ Contam Toxicol 41(3):371–377. https://doi.org/10.1007/BF01688881
Yang X, Shang C (2005) Quantification of aqueous cyanogen chloride and cyanogen bromide in environmental samples by MIMS. Water Res 39(9):1709–1718. https://doi.org/10.1016/j.watres.2005.01.030
Srivastava A, Adesina NO (2022) Chapter 1 - Graphene—technology and integration with semiconductor electronics. Theor Comput Chem 21:1–40. https://doi.org/10.1016/B978-0-12-819514-7.00006-3
Cho B, Yoon J, Hahm MG, Kim D-H, Kim AR, Kahng YH, Park S-W, Lee Y-J, Park S-G, Kwon J-D, Kim CS, Song M, Jeong Y, Nam K-S, Ko HC (2014) Graphene-based gas sensor: metal decoration effect and application to a flexible device. J Mater Chem C 2(27):5280–5285. https://doi.org/10.1039/C4TC00510D
Liu L, Zhang J, Gao H, Wang L, Jiang X, Zhao J (2016) Tailoring physical properties of graphene: effects of hydrogenation, oxidation, and grain boundaries by atomistic simulations. Comput Mater Sci 112:527–546. https://doi.org/10.1016/j.commatsci.2015.06.032
Septiani NLW, Yuliarto B (2016) Review—the development of gas sensor based on carbon nanotubes. J Electrochem Soc 163(3):B97. https://doi.org/10.1149/2.0591603jes
Bai H, Shi G (2007) Gas sensors based on conducting polymers. Sensors 7(3):267–307
Govindaraju KM, Prakash C (2014) Synthesis of zinc modified poly(aniline-co-pyrrole) coatings and its anti-corrosive performance on low nickel stainless steel. Colloids Surf A Physicochem Eng Asp 465. https://doi.org/10.1016/j.colsurfa.2014.10.011
Ahmad S, Bibi S, Bilal S, A-u-H S, Ayub K (2015) Spectral and electronic properties of p-conjugated oligomers and polymers of poly (o-chloroaniline-co-o-toluidine) calculated with density functional theory. Synth Met 205:153. https://doi.org/10.1016/j.synthmet.2015.04.005
Abdelhamid ME, O’Mullane AP, Snook GA (2015) Storing energy in plastics: a review on conducting polymers & their role in electrochemical energy storage. RSC Adv 5(15):11611–11626. https://doi.org/10.1039/C4RA15947K
Snook G, Bhatt A, Abdelhamid M, Best A (2012) Role of H+ in polypyrrole and poly(3,4-ethylenedioxythiophene) formation using FeCl3•6H2O in the room temperature ionic liquid, C4mpyrTFSI. Aust J Chem 65:1513–1522. https://doi.org/10.1071/CH12322
Otero TF (2013) Biomimetic conducting polymers: synthesis, materials, properties, functions, and devices. Polym Rev 53(3):311–351. https://doi.org/10.1080/15583724.2013.805772
Saha J, Hossain M, Ghosh M (2019) DFT study of response mechanism and selectivity of poly(3,4-ethylenedioxythiophene) towards CO2 and SO2 as gas sensor. Struct Chem 30. https://doi.org/10.1007/s11224-018-1272-4
Mandú L, Batagin-Neto A (2018) Chemical sensors based on N-substituted polyaniline derivatives: reactivity and adsorption studies via electronic structure calculations. J Mol Model 24. https://doi.org/10.1007/s00894-018-3660-5
Ullah H, A-u-H S, Bilal S, Ayub K (2013) DFT study of polyaniline NH3, CO2 and CO gas sensors: comparison with recent experimental data. J Phys Chem C 117:23701. https://doi.org/10.1021/jp407132c
Coleone A, Barboza B, Batagin-Neto A (2021) Polypyrrole derivatives for detection of toxic gases: a theoretical study. Polyme Adv Technol 32. https://doi.org/10.1002/pat.5449
Ratcliffe NM (1990) Polypyrrole-based sensor for hydrazine and ammonia. Anal Chim Acta 239:257–262. https://doi.org/10.1016/S0003-2670(00)83859-3
Becke AD (1993) A new mixing of Hartree-Fock and local density-functional theories. J Chem Phys 98(2):1372–1377. https://doi.org/10.1063/1.464304
Maksymiuk K (2006) Chemical reactivity of polypyrrole and its relevance to polypyrrole based electrochemical sensors. Electroanalysis 18:1537–1551. https://doi.org/10.1002/elan.200603573
Radhakrishnan S, Deshpande SD (2002) Conducting polymers functionalized with phthalocyanine as nitrogen dioxide sensors. Sensors 2(5):185–194
Beheshtian J, Baei MT, Ahmadi Peyghan A (2012) Theoretical study of CO adsorption on the surface of BN, AlN, BP and AlP nanotubes. Surf Sci 606:981–985. https://doi.org/10.1016/j.susc.2012.02.019
Hosseini S, Entezami A (2003) Conducting polymer blends of polypyrrole with polyvinyl acetate, polystyrene, and polyvinyl chloride based toxic gas sensors. J Appl Polym Sci 90:49–62. https://doi.org/10.1002/app.12492
Shokuhi Rad A, Esfahanian M, Ganjian E, Tayebi HA, Novir SB (2016) The polythiophene molecular segment as a sensor model for H2O, HCN, NH3, SO3, and H2S: a density functional theory study. J Mol Model 22(6):127. https://doi.org/10.1007/s00894-016-3001-5
Rad A, Valipour P, Gholizade A, Mousavinezhad S (2015) Interaction of SO2 and SO3 on terthiophene (as a model of polythiophene gas sensor): DFT calculations. Chem Phys Lett 639. https://doi.org/10.1016/j.cplett.2015.08.062
Shokuhi Rad A, Valipour P (2015) Interaction of methanol with some aniline and pyrrole derivatives: DFT calculations. Synth Met 209:502–511. https://doi.org/10.1016/j.synthmet.2015.08.021
Shokuhi Rad A (2015) Application of polythiophene to methanol vapor detection: an ab initio study. J Mol Model 21(11):285. https://doi.org/10.1007/s00894-015-2832-9
Rad AS (2016) Terthiophene as a model sensor for some atmospheric gases: theoretical study. Mol Phys 114(5):584–591. https://doi.org/10.1080/00268976.2015.1102348
Rad A, Ghasemi ateni S, Tayebi H, Valipour P, Foukolaei V, (2016) First-principles DFT study of SO2 and SO3 adsorption on 2PANI: a model for polyaniline response. J Sulfur Chem 37. https://doi.org/10.1080/17415993.2016.1170834
Rad AS, Esfahanian M, Ganjian E, Tayebi H-a (2016) Ab-initio study of physisorption of hydrogen cyanide on 2PANI: a model for polyaniline gas sensor. Z Phys Chem 230(10):1487–1498. https://doi.org/10.1515/zpch-2015-0645
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06 functionals and 12 other functionals. Theoret Chem Acc 119(5):525–525. https://doi.org/10.1007/s00214-007-0401-8
Ivanov P (2021) Computational study (MM and DFT) on the conformations of some aromatic crown ether rotaxane macrocycles. Comput Theor Chem 1203:113266. https://doi.org/10.1016/j.comptc.2021.113266
Cohen AJ, Mori-Sánchez P, Yang W (2012) Challenges for density functional theory. Chem Rev 112(1):289–320. https://doi.org/10.1021/cr200107z
Lin YS, Li GD, Mao SP, Chai JD (2013) Long-range corrected hybrid density functionals with improved dispersion corrections. J Chem Theory Comput 9(1):263–272. https://doi.org/10.1021/ct300715s
Josa D, Rodríguez-Otero J, Cabaleiro-Lago EM, Rellán-Piñeiro M (2013) Analysis of the performance of DFT-D, M05–2X and M06–2X functionals for studying π⋯π interactions. Chem Phys Lett 557:170–175. https://doi.org/10.1016/j.cplett.2012.12.017
Masoodi H, Bagheri S, Ranjbar-Karimi R (2016) Theoretical prediction of some novel nanotubes composed of macrocyclic structures: a DFT study. Chem Phys Lett 667. https://doi.org/10.1016/j.cplett.2016.11.016
Zhan C-G, Nichols JA, Dixon DA (2003) Ionization potential, electron affinity, electronegativity, hardness, and electron excitation energy: molecular properties from density functional theory orbital energies. J Phys Chem A 107(20):4184–4195. https://doi.org/10.1021/jp0225774
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Petersson GA, Nakatsuji H, Li X, Caricato M, Marenich AV, Bloino J, Janesko BG, Gomperts R, Mennucci B, Hratchian HP, Ortiz JV, Izmaylov AF, Sonnenberg JL, Williams, Ding F, Lipparini F, Egidi F, Goings J, Peng B, Petrone A, Henderson T, Ranasinghe D, Zakrzewski VG, Gao J, Rega N, Zheng G, Liang W, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Throssell K, Montgomery Jr. JA, Peralta JE, Ogliaro F, Bearpark MJ, Heyd JJ, Brothers EN, Kudin KN, Staroverov VN, Keith TA, Kobayashi R, Normand J, Raghavachari K, Rendell AP, Burant JC, Iyengar SS, Tomasi J, Cossi M, Millam JM, Klene M, Adamo C, Cammi R, Ochterski JW, Martin RL, Morokuma K, Farkas O, Foresman JB, Fox DJ (2016) Gaussian 16 Rev C.01. Wallingford, CT
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33(5):580–592. https://doi.org/10.1002/jcc.22885
Chen S, Kar T (2012) Theoretical investigation of inhibition efficiencies of some Schiff bases as corrosion inhibitors of aluminum. Int J Electrochem Sci 7:6265–6275
Barabás J, Vanbuel J, Ferrari P, Janssens E, Höltzl T (2019) Non-covalent interactions and charge transfer between propene and neutral yttrium-doped and pure gold clusters. Chemistry (Weinheim an der Bergstrasse, Germany) 25(69):15795–15804. https://doi.org/10.1002/chem.201902794
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122(45):11154–11161. https://doi.org/10.1021/ja0017864
Koch U, Popelier PLA (1995) Characterization of C-H-O hydrogen bonds on the basis of the charge density. J Phys Chem 99(24):9747–9754. https://doi.org/10.1021/j100024a016
Ganji M, Tavassoli H, Alamol-hoda R, Mehdizadeh M (2018) First-principles and molecular dynamics simulation studies of functionalization of Au32 golden fullerene with amino acids. Sci Rep 8. https://doi.org/10.1038/s41598-018-29887-5
Hashemkhani Shahnazari G, Darvish Ganji M (2021) Understanding structural and molecular properties of complexes of nucleobases and Au13 golden nanocluster by DFT calculations and DFT-MD simulation. Sci Rep 11(1):435. https://doi.org/10.1038/s41598-020-80161-z
Niknam P, Jamehbozorgi S, Rezvani M, Izadkhah V (2022) Understanding delivery and adsorption of Flutamide drug with ZnONS based on: dispersion-corrected DFT calculations and MD simulations. Physica E 135:114937. https://doi.org/10.1016/j.physe.2021.114937
Jacobsen H (2008) Localized-orbital locator (LOL) profiles of chemical bonding. Can J Chem 86(7):695–702. https://doi.org/10.1139/v08-052
Schmider HL, Becke AD (2000) Chemical content of the kinetic energy density. J Mol Struct (Thoechem) 527(1):51–61. https://doi.org/10.1016/S0166-1280(00)00477-2
Peng S, Cho K, Qi P, Dai H (2004) Ab initio study of CNT NO2 gas sensor. Chem Phys Lett 387:271–276. https://doi.org/10.1016/j.cplett.2004.02.026
Acknowledgements
The authors gratefully acknowledge TWAS for their generous support.
Funding
This study received support from the Research Grant (no. 21–234 RG/CHE/AS_I-FR3240319472) provided by The World Academy of Sciences (TWAS). Additionally, this work was also supported by a research grant from Jagannath University.
Author information
Authors and Affiliations
Contributions
Urmi Khanom performed simulations for data collection and wrote the manuscript draft. Joyanta K. Saha was responsible for conceptualizing, and validating the methodology and writing the original manuscript. Joonkyung Jang and Mahmudur Rahman: reviewed the manuscript
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khanom, U., Saha, J.K., Jang, J. et al. Revealing conducting organic polymers’ interaction with cyanogen halides: DFT insights for enhanced gas sensing applications. Struct Chem (2024). https://doi.org/10.1007/s11224-023-02275-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-023-02275-0