Abstract
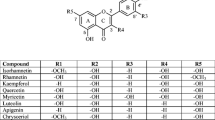
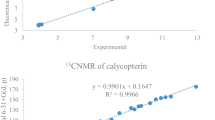
In the present research, the physicochemical properties including electronic and structural properties and antioxidant activity of six fluorinated flavone derivatives have been discussed using of density functional theory calculations. Benchmarking studies of the DFT-B3LYP/6–311 + G(d, p) computational method were done using experimental 1HNMR and 13CNMR data of flavones 2 and 5. The experimental and calculated spectral data are in very good agreement with R2 < 92%. Using the DFT-B3LYP/6–311 + G(d, p) method, bond lengths and bond angles, intramolecular hydrogen interactions, EHOMO and ELUMO, electrophilicity index, and reactivity against active oxygen radicals were investigated. The local electrophilicity indices were calculated for flavones 1–6. Three reactive centers can be considered for nucleophilic attack on flavones 1–6. The data showed that the most active center is C9 on the 4-pyrone ring, conducting the Michael reaction, and the presence of -CF3 group on these compounds increases the electrophilic properties of flavones 1–6. Using three mechanisms of hydrogen atom transfer (HAT), single electron transfer followed by proton transfer (SET-PT), and the sequential proton loss electron transfer (SPLET) mechanisms, antioxidant activity of the studied flavones was investigated and compared with data for phenol. The presence of intramolecular hydrogen bonds strongly affects the antioxidant properties. According to the above-mentioned mechanisms, antioxidant activity of studied flavones 1–6 is higher than that of phenol. The presence of fluorine atom has a significant effect on their physicochemical properties.














Similar content being viewed by others
Availability of data and material
Not applicable.
References
Salaj N, Kladar N, Čonić BS, Jeremić K, Hitl M, Gavarić N, Božin B (2021) Traditional multi-herbal formula in diabetes therapy—antihyperglycemic and antioxidant potential. Arab J Chem 14:103347. https://doi.org/10.1016/j.arabjc.2021.103347
Farag RS, Abdel-Latif MS, Abd El Baky HH, Tawfeek LS (2020) Phytochemical screening and antioxidant activity of some medicinal plants’ crude juices. Biotech Reports 28:e00536. https://doi.org/10.1016/j.btre.2020.e00536
Sarmiento-Salinas FL, Perez-Gonzalez A, Acosta-Casique A, Ix-Ballote A, Diaz A, Treviño S, Rosas-Murrieta NH, Millán-Perez-Peña L, Maycotte P (2021) Reactive oxygen species: Role in carcinogenesis, cancer cell signaling and tumor progression. Life Sci 284:119942. https://doi.org/10.1016/j.lfs.2021.119942
Faller ALK, Fialho E (2010) Polyphenol content and antioxidant capacity in organic and conventional plant foods. J Food Compos Anal 23:561–568. https://doi.org/10.1016/j.jfca.2010.01.003
Santhakumar AB, Battino M, Alvarez-Suarez JM (2018) Dietary polyphenols: structures, bioavailability and protective effects against atherosclerosis. Food Chem Toxicol 113:49–65. https://doi.org/10.1016/j.fct.2018.01.022
Sheng H, Sun X, Yan Y, Yuan Q, Wang J, Shen X (2020) Metabolic engineering of microorganisms for the production of flavonoids. Front Bioeng Biotechnol 8:589069. https://doi.org/10.3389/fbioe.2020.589069
Williams RJ, Spencer JP, Rice-Evans C (2004) Flavonoids: antioxidants or signaling molecules? Free Radic Biol Med 36:838–849. https://doi.org/10.1016/j.freeradbiomed.2004.01.001
Banjarnahor SD, Artanti N (2014) Antioxidant properties of flavonoids. Med J Indones 23:239–44. https://doi.org/10.13181/mji.v23i4.1015
Dimić D, Milenković D, Ilić J, Šmit B, Amić A, Marković Z, Marković JD (2018) Experimental and theoretical elucidation of structural and antioxidant properties of vanillylmandelic acid and its carboxylate anion. Spectrochim Acta A Mol Biomol Spectrosc 198:61–70. https://doi.org/10.1016/j.saa.2018.02.063
Dimić D, Milenković D, Marković JD, Marković Z (2017) Antiradical activity of catecholamines and metabolites of dopamine: theoretical and experimental study. Phys Chem Chem Phys 19:12970–12980. https://doi.org/10.1039/C7CP01716B
Johnson BM, Shu YZ, Zhuo X, Meanwell NA (2020) Metabolic and pharmaceutical aspects of fluorinated compounds. J Med Chem 63:6315–6386. https://doi.org/10.1021/acs.jmedchem.9b01877
Ismail FM (2002) Important fluorinated drugs in experimental and clinical use. J Fluor Chem 118:27–33. https://doi.org/10.1016/S0022-1139(02)00201-4
Kirk KL (2006) Fluorine in medicinal chemistry: recent therapeutic applications of fluorinated small molecules. J Fluor Chem 127:1013–1029. https://doi.org/10.1016/j.jfluchem.2006.06.007
Gouverneur V, Seppelt K (2015) Introduction: fluorine chemistry. Chem Reviews 115:563–565. https://doi.org/10.1021/cr500686k
Shams B, Saeidian H (2018) Design of the novel neutral organic superacids by comprehensive DFT study on organic fluorosulfuric acids, Comput. Theor Chem 1135:48–55. https://doi.org/10.1016/j.comptc.2018.05.011
Saeidian H, Sadighian H, Abdoli M, Sahandi M (2017) Versatile and green synthesis, spectroscopic characterizations, crystal structure and DFT calculations of 1, 2, 3-triazole-based sulfonamides. J Mol Struct 1131:73–78. https://doi.org/10.1016/j.molstruc.2016.11.027
Khademloo E, Kadhodaeian HA, Jameie SB, Farhadi M, Saeidian H (2022) A detailed density functional theory investigation on physicochemical properties of ciclopirox derivatives: a potential candidate for prevention of age-related macular degeneration. J Mol Struct 1268:133678. https://doi.org/10.1016/j.molstruc.2022.133678
Menichincheri M, Ballinari D, Bargiotti A, Bonomini L, Ceccarelli W, D’Alessio R, Fretta A, Moll J, Polucci P, Soncini C, Tibolla M, TrossetJ, Vanotti E (2004) Catecholic flavonoids acting as telomerase inhibitors. J Med Chem 47:6466–6475. https://doi.org/10.1021/jm040810b
Alshammari, M. Synthesis of fluoroflavones as potential neuroprotective agents, (2019), https://egrove.olemiss.edu/etd/1587
Frisch JM et al (2013) Gaussian 09, Gaussian, Inc, Wallingford CT. Gaussian, Inc
Mennucci B (2012) Polarizable continuum model. Wiley Interdiscip Rev Comput Mol Sci 2:386–404. https://doi.org/10.1002/wcms.1086
Raczyńska ED (2019) Application of the extended HOMED (harmonic oscillator model of aromaticity) index to simple and tautomeric five-membered heteroaromatic cycles with C, N, O, P, and S atoms. Symmetry 11:146. https://doi.org/10.3390/sym11020146
Pal R, Chattaraj PK (2023) Electrophilicity index revisited. J Comput Chem 44:278–297. https://doi.org/10.1002/jcc.26886
Robles A, Franco-Pérez M, Gázquez JL, Cárdenas C, Fuentealba P (2018) Local electrophilicity. J Mol Model 24:1–12. https://doi.org/10.1007/s00894-018-3785-6
Szeląg M, Urbaniak A, Bluyssen HA (2014) A theoretical antioxidant pharmacophore for natural hydroxycinnamic acids. Open Chem 13:17–31. https://doi.org/10.1515/chem-2015-0001
Rimarčík J, Lukeš V, Klein E, Ilčin M (2010) Study of the solvent effect on the enthalpies of homolytic and heterolytic N-H bond cleavage in p-phenylenediamine and tetracyano-p-phenylenediamine. J Mol Struct 952:25–30. https://doi.org/10.1016/j.theochem.2010.04.002
Marković Z, Tošović J, Milenković D, Marković S (2016) Revisiting the solvation enthalpies and free energies of the proton and electron in various solvents. Comput Theor Chem 1077:11–17. https://doi.org/10.1016/j.comptc.2015.09.007
Mazurek AH, Szeleszczuk L, Pisklak DM (2020) Periodic DFT calculations—review of applications in the pharmaceutical sciences. Pharm 12:415. https://doi.org/10.3390/pharmaceutics12050415
Taghiboroujerdi M, Mirjafary Z, Saeidian H (2023) Aromaticity enhancement of cyclopentadiene in piperidine derivatives: a DFT study on combination of the anomeric effect and Schleyer hyperconjugative aromaticity. New J Chem 47:5863–5874. https://doi.org/10.1039/D3NJ00337J
Jasiński R (2018) β-Trifluoromethylated nitroethenes in Diels-Alder reaction with cyclopentadiene: a DFT computational study. J Fluor Chem 206:1–7. https://doi.org/10.1016/j.jfluchem.2017.12.008
Bursch M, Mewes JM, Hansen A, Grimme S (2022) Best‐practice DFT protocols for basic molecular computational chemistry. Angew Chem Int Ed 61:e202205735. https://doi.org/10.1002/anie.202205735
Wolinski K, Hinton JF, Pulay P (1990) Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J Am Chem Soc 112:8251–8260. https://doi.org/10.1021/ja00179a005
Avdović EH, Dimić DS, Marković JMD, Vuković N, Radulović MD, Živanović MN, Filipović ND, Đorović JR, Trifunović RS, Marković ZS (2019) Spectroscopic and theoretical investigation of the potential anti-tumor and anti-microbial agent, 3-(1-((2-hydroxyphenyl) amino) ethylidene) chroman-2, 4-dione. Spectrochim Acta A Mol Biomol Spectrosc 206:421–429. https://doi.org/10.1016/j.saa.2018.08.034
Michalík M, Poliak P, Lukeš V, Klein E (2019) From phenols to quinones: thermodynamics of radical scavenging activity of para-substituted phenols. Phytochem 166:112077. https://doi.org/10.1016/j.phytochem.2019.112077
Boulebd H (2020) Theoretical insights into the antioxidant activity of moracin T. Free Radical Res 54:221–230. https://doi.org/10.1080/10715762.2020.1747616
Wang G, Liu Y, Zhang L, An L, Chen R, Liu Y, Luo Q, Li Y, Wang H, Xue Y (2020) Computational study on the antioxidant property of coumarin-fused coumarins. Food Chem 304:125446. https://doi.org/10.1016/j.foodchem.2019.125446
Leopoldini M, Russo N, Toscano M (2011) The molecular basis of working mechanism of natural polyphenolic antioxidants. Food Chem 125:288–306. https://doi.org/10.1016/j.foodchem.2010.08.012
Krygowski TM, Cyrański MK (2001) Structural aspects of aromaticity. Chem Rev 101:1385–1420. https://doi.org/10.1021/cr990326u
Chen Z, Wannere CS, Corminboeuf C, Puchta R, Schleyer PVR (2005) Nucleus-independent chemical shifts (NICS) as an aromaticity criterion. Chem Rev 105:3842–3888. https://doi.org/10.1021/cr030088
Suresh CH, Remya GS, Anjalikrishna PK (2022) Molecular electrostatic potential analysis: a powerful tool to interpret and predict chemical reactivity. Wiley Interdiscip Rev Comput Mol Sci 12:e1601. https://doi.org/10.1002/wcms.1601
Weinhold F, Landis CR, Glendening ED (2016) What is NBO analysis and how is it useful? Int Rev Phys Chem 35:399–440. https://doi.org/10.1080/0144235X.2016.1192262
Pérez P, Domingo LR, Aizman A, Contreras R (2007) The electrophilicity index in organic chemistry, Comput. Theor Chem 19:139–201. https://doi.org/10.1016/S1380-7323(07)80010-0
Lončar A, Negrojević L, Dimitrić-Marković J, Dimić D (2021) The reactivity of neurotransmitters and their metabolites towards various nitrogen-centered radicals: experimental, theoretical, and biotoxicity evaluation. Comput Biol Chem 95:107573. https://doi.org/10.1016/j.compbiolchem.2021.107573
Santos-Sánchez NF, Salas-Coronado R, Villanueva-Cañongo C, Hernández-Carlos B (2019) Antioxidant compounds and their antioxidant mechanism. Antioxidants 10:1–29. https://doi.org/10.5772/intechopen.85270
Funding
None.
Author information
Authors and Affiliations
Contributions
Hamid Saeidian: Conceptualization, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing- review & editing. Azadeh Bakhtiari: Formal analysis, Investigation, Resources, Software, Visualization. Zohreh Mirjafary: Conceptualization, Formal analysis, Investigation, Resources, Software, Validation, Visualization, Writing- review & editing. Kambiz Larijani: Advising, Writing-review & editing.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saeidian, H., Bakhtiari, A., Mirjafary, Z. et al. DFT investigation of physicochemical and antioxidant properties of fluorinated flavones. Struct Chem (2023). https://doi.org/10.1007/s11224-023-02258-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11224-023-02258-1