Abstract
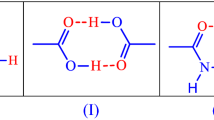
Two pharmaceutical salts of paliperidone, namely, paliperidone benzoate (PLPT·BA) and paliperidone salicylate (PLPT·SA), were successfully synthesized using benzoic acid (BA) and salicylic acid (SA) as starting materials through a solvent evaporation method. Our study rectified the misidentification of PLPT·BA as a co-crystal in previous research by providing single-crystal structure data and further analysis. The salts’ structures were confirmed through ΔpKa calculations, single-crystal x-ray diffraction (SCXRD) analysis, powder x-ray diffraction (PXRD), and infrared (IR) spectroscopy, while their solubility was also evaluated. Moreover, significant enhancements in thermal stability were observed for both PLPT·BA and PLPT·SA, with increases of 39 K and 32 K, respectively, in their decomposition temperatures compared to pure PLPT. Additionally, intramolecular charge-assisted hydrogen bonds (N+–H···O) were found in both salts, which crystallized in the monoclinic system with the \(P\overline{1}\) P-1 (2) space group. Furthermore, solubility and dissolution rate experiments indicated slight improvements in their solubility and dissolution rate compared to PLPT. This pioneering research provides crucial data on the single-crystal structures and thermal properties of PLPT·BA and PLPT·SA, laying a foundation for further investigations into their potential applications in drug formulations and pharmacology and optimizing drug formulations for enhanced clinical efficacy.










Similar content being viewed by others
Data availability
No data was used for the research described in the article.
References
Dickson MC, Nguyen MM, Patel C, Grabich SC, Benson C, Cothran T, Skrepnek GH (2023) Adherence, persistence, readmissions, and costs in medicaid members with schizophrenia or schizoaffective disorder initiating paliperidone palmitate versus switching oral antipsychotics: a real-world retrospective investigation. Adv Ther 40(1):349–366. https://doi.org/10.1007/s12325-022-02354-4
Giron-Hernandez C, Han JH, Alberio R, Singh A, Garcia-Portilla MP, Pompili M, Knight RK, Richarz U, Gopal S, Antunes J (2023) Efficacy and safety of paliperidone palmitate 6-month versus paliperidone palmitate 3-month long-acting injectable in European patients with schizophrenia: a post hoc analysis of a global phase-3 double-blind randomized non-inferiority study. Neuropsychiatr Dis Treat 19:895–906. https://doi.org/10.2147/NDT.S400342
Manini G, Benali S, Mathew A, Napolitano S, Raquez JM, Goole J (2022) Paliperidone palmitate as model of heat-sensitive drug for long-acting 3D printing application. Int J Pharm 618:121662. https://doi.org/10.1016/j.ijpharm.2022.121662
Rehman S, Nabi B, Javed A, Khan T, Iqubal A, Ansari MJ, Baboota S, Ali J (2022) Unraveling enhanced brain delivery of paliperidone-loaded lipid nanoconstructs: pharmacokinetic, behavioral, biochemical, and histological aspects. Drug Deliv 29(1):1409–1422. https://doi.org/10.1080/10717544.2022.2069880
Xu Z, Liu T, Jiang Y, Chen Z, Shi X, Xu Y, Yu N, Hua X, Liang XJ, Yuan X, Guo S (2022) Microcrystals of ketal-linked paliperidone prodrugs for long-acting antipsychotics. Mol Pharm 19(11):3846–3857. https://doi.org/10.1021/acs.molpharmaceut.2c00348
Allott K, Yuen HP, Baldwin L, O’Donoghue B, Fornito A, Chopra S, Nelson B, Graham J, Kerr MJ, Proffitt TM, Ratheesh A, Alvarez-Jimenez M, Harrigan S, Brown E, Thompson AD, Pantelis C, Berk M, McGorry PD, Francey SM, Wood SJ (2023) Effects of risperidone/paliperidone versus placebo on cognitive functioning over the first 6 months of treatment for psychotic disorder: secondary analysis of a triple-blind randomised clinical trial. Transl Psychiatry 13(1):199. https://doi.org/10.1038/s41398-023-02501-7
Cai R, Decuypere F, Chevalier P, Desseilles M, Lambert M, Fakra E, Wimmer A, Guillon P, Pype S, Godet A, Borgmeier V (2022) Assessment of risk factors of treatment discontinuation among patients on paliperidone palmitate and risperidone microspheres in France. Germany and Belgium BMC Psychiatry 22(1):382. https://doi.org/10.1186/s12888-022-03914-2
Hieronymus F, Correll CU, Ostergaard SD (2023) Initial severity of the Positive and Negative Syndrome Scale (PANSS)-30, its main subscales plus the PANSS-6, and the relationship to subsequent improvement and trial dropout: a pooled participant-level analysis of 18 placebo-controlled risperidone and paliperidone trials. Transl Psychiatry 13(1):191. https://doi.org/10.1038/s41398-023-02491-6
Ibrahim HG, Malcolm BJ, Gogineni HP (2021) Assessing outcomes between risperidone microspheres and paliperidone palmitate long-acting injectable antipsychotics among veterans. Fed Pract 38(12):586–591. https://doi.org/10.12788/fp.0195
Mahdy WYB, Yamamoto K, Ito T, Fujiwara N, Fujioka K, Horai T, Otsuka I, Imafuku H, Omura T, Iijima K, Yano I (2023) Physiologically-based pharmacokinetic model to investigate the effect of pregnancy on risperidone and paliperidone pharmacokinetics: application to a pregnant woman and her neonate. Clin Transl Sci 16(4):618–630. https://doi.org/10.1111/cts.13473
Suzuki H, Hibino H (2022) Comparison of treatment retention between risperidone long-acting injection, paliperidone palmitate, and aripiprazole once-monthly in elderly patients with schizophrenia. Psychogeriatrics 22(1):159–160. https://doi.org/10.1111/psyg.12784
Zhao M, Qin B, Mao Y, Zhang Y, Zhao R, Wang A, Wang H, Zhao J, Wang C (2023) Paliperidone palmitate versus risperidone long-acting injectable in patients with schizophrenia: a meta-analysis of efficacy and safety. Neuropsychiatr Dis Treat 19:749–757. https://doi.org/10.2147/NDT.S407259
Muthu MS, Sahu AK, Sonali AA, Kaklotar D, Rajesh CV, Singh S, Pandey BL (2016) Solubilized delivery of paliperidone palmitate by D-alpha-tocopheryl polyethylene glycol 1000 succinate micelles for improved short-term psychotic management. Drug Deliv 23(1):230–237. https://doi.org/10.3109/10717544.2014.909907
Nagata S, Jin-nai A, Hirai K, Baba M, Higashi K, Tanaka Y (2013) Evaluation of dissolution of osmotic-controlled release paliperidone tablets using the reciprocating cylinder method. Yakugaku Zasshi 133(3):405–410. https://doi.org/10.1248/yakushi.12-00259
Subhani S, Lukacova V, Kim C, Rodriguez-Vera L, Muniz P, Rodriguez M, Cristofoletti R, Van Os S, Suarez E, Schmidt S, Vozmediano V (2023) Leveraging physiologically based modelling to provide insights on the absorption of paliperidone extended-release formulation under fed and fasting conditions. Pharmaceutics 15(2). https://doi.org/10.3390/pharmaceutics15020629
Yu Y, Ngo HV, Jin G, Tran PHL, Tran TTD, Nguyen VH, Park C, Lee BJ (2021) Double-controlled release of poorly water-soluble paliperidone palmitate from self-assembled albumin-oleic acid nanoparticles in PLGA in situ forming implant. Int J Nanomedicine 16:2819–2831. https://doi.org/10.2147/IJN.S302514
Aakeroy CB, Wijethunga TK, Desper J (2015) Crystal engineering of energetic materials: co-crystals of ethylenedinitramine (EDNA) with modified performance and improved chemical stability. Chemistry 21(31):11029–11037. https://doi.org/10.1002/chem.201501721
Alhalaweh A, Roy L, Rodriguez-Hornedo N, Velaga SP (2012) pH-dependent solubility of indomethacin-saccharin and carbamazepine-saccharin cocrystals in aqueous media. Mol Pharm 9(9):2605–2612. https://doi.org/10.1021/mp300189b
Babu NJ, Sanphui P, Nangia A (2012) Crystal engineering of stable temozolomide cocrystals. Chem Asian J 7(10):2274–2285. https://doi.org/10.1002/asia.201200205
Butreddy A, Sarabu S, Bandari S, Dumpa N, Zhang F, Repka MA (2020) Polymer-assisted aripiprazole-adipic acid cocrystals produced by hot melt extrusion techniques. Cryst Growth Des 20(7):4335–4345. https://doi.org/10.1021/acs.cgd.0c00020
Chen YM, Rodriguez-Hornedo N (2018) Cocrystals mitigate negative effects of high pH on solubility and dissolution of a basic drug. Cryst Growth Des 18(3):1358–1366. https://doi.org/10.1021/acs.cgd.7b01206
Gao Y, Gao J, Liu Z, Kan H, Zu H, Sun W, Zhang J, Qian S (2012) Coformer selection based on degradation pathway of drugs: a case study of adefovir dipivoxil-saccharin and adefovir dipivoxil-nicotinamide cocrystals. Int J Pharm 438(1–2):327–335. https://doi.org/10.1016/j.ijpharm.2012.09.027
Jin S, Haskins MM, Deng CH, Matos C, Zaworotko MJ (2023) Crystal engineering of ionic cocrystals comprising Na/K salts of hesperetin with hesperetin molecules and solubility modulation. IUCrJ 10(Pt 3):329–340. https://doi.org/10.1107/S205225252300266X
Kuminek G, Rodriguez-Hornedo N, Siedler S, Rocha HV, Cuffini SL, Cardoso SG (2016) How cocrystals of weakly basic drugs and acidic coformers might modulate solubility and stability. Chem Commun (Camb) 52(34):5832–5835. https://doi.org/10.1039/c6cc00898d
Xing C, Chen T, Wang L, An Q, Jin Y, Yang D, Zhang L, Du G, Lu Y (2022) Two novel co-crystals of naproxen: comparison of stability, solubility and intermolecular interaction. Pharmaceuticals (Basel) 15(7). https://doi.org/10.3390/ph15070807
Zhang ZH, Zhang Q, Zhang QQ, Chen C, He MY, Chen Q, Song GQ, Xuan XP, Huang XF (2015) From a binary salt to salt co-crystals of antibacterial agent lomefloxacin with improved solubility and bioavailability. Acta Crystallogr B Struct Sci Cryst Eng Mater 71(Pt 4):437–446. https://doi.org/10.1107/S2052520615011191
Radha-Rani E, Venkata-Radha G (2021) Engineering cocrystals of paliperidone with enhanced solubility and dissolution characteristics. Arhiv za farmaciju 71(5):393–409. https://doi.org/10.5937/arhfarm71-32997
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Crystallogr 42(2):339–341. https://doi.org/10.1107/S0021889808042726
Sheldrick G (2008) A short history of SHELX. Acta Crystallogr, Sect A: Found Adv 64(1):112–122. https://doi.org/10.1107/S0108767307043930
Xie YF, Yuan PH, Heng TY, Du LD, An Q, Zhang BX, Zhang L, Yang DZ, Du GH, Lu Y (2022) Insight into the formation of cocrystal and salt of tenoxicam from the isomer and conformation. Pharmaceutics 14(9). https://doi.org/10.3390/pharmaceutics14091968
Quintano M, Moura RT, Kraka E (2023) The pKa rule in light of local mode force constants. Chem Phys Lett 826. https://doi.org/10.1016/j.cplett.2023.140654
Powers K, Geiger D (2018) Salt formation vs. co-crystallization: an exploration of the Delta pKa rule for a series of aromatic acids and bases. Abstr Pap Am Chem S 255
Lusi M, Kavanagh O (2019) Controlling the salt-cocrystal continuum and pKa rule: the multi-drug ionic-cocrystals of lamatrigine and valproic acid. Acta Crystallogr A 75. https://doi.org/10.1107/S2053273319089678
Kumar S, Nanda A (2018) Approaches to design of pharmaceutical cocrystals: a review. Mol Cryst Liq Cryst 667(1):54–77. https://doi.org/10.1080/15421406.2019.1577462
Acknowledgements
The authors would like to thank Yanbo times (www.yanbotimes.cn) for the support of TGA and DSC.
Funding
This work was supported by the National Nature Science Foundation of China (grant number 22201134) and the Natural Science Foundation for Colleges and Universities of Jiangsu Province (grant number 22KJB150028).
Author information
Authors and Affiliations
Contributions
Zheng Shen: Growth of crystals, draft validation and resources. Jian Chen: writing, drawing, review and editing. Jilong Ge: TGA-DSC experiments. Zhuoer Cai: single crystal analysis. Xiu-Ni Hua and Baiwang Sun: supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shen, Z., Chen, J., Ge, J. et al. Crystal structures and properties of two aromatic carboxylic acid-based medicinal salts of paliperidone. Struct Chem 35, 967–975 (2024). https://doi.org/10.1007/s11224-023-02247-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02247-4