Abstract
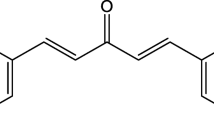
Oxidative stress driven by the accumulation of free radicals and reactive oxygen species (ROS) in the human body is a key contributor to various diseases. Curcumin, a polyphenolic compound derived from turmeric, has garnered attention for its antioxidant potential. In this context, a recent experimental study by Hao et al. introduced curcumin derivatives with incorporated electron-donating groups (allyl and isopentenyl), aiming to enhance antioxidant activity while circumventing the limitations of traditional curcumin. Building upon this experimental foundation, our study employs computational techniques (DFT) to unravel the molecular mechanisms underpinning the superior antioxidant effects observed in these novel derivatives. We investigated three prominent antioxidant mechanisms: hydrogen atom transfer (HAT), single electron transfer-proton transfer (SET-PT), and sequential proton loss electron transfer (SPLET). Our results reveal that the allyl and isopentenyl groups contribute in enhancing the antioxidant properties of the derivatives, as evidenced by reduced energies of most of thermodynamic parameters. Moreover, the analysis of highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) energies indicates their enhanced reactivity. Notably, the computational investigation of spin densities validates the radical scavenging potential of these derivatives. Our findings suggest that the strategically designed derivatives exhibit powerful antioxidant properties, positioning them as promising candidates for further therapeutic applications. This comprehensive study bridges experimental findings with computational insights to unravel the intricate molecular mechanisms driving the enhanced antioxidant efficacy of the newly developed curcumin derivatives.





Similar content being viewed by others
References
Malik Al-Rubaei ZM, Mohammad TU, Karim Ali L (2014) Pak J Biol Sci 17:1237–1241
Suryanarayana P, Satyanarayana A, Balakrishna N, Kumar PU, Reddy GB (2007) Med Sci Monit 13:BR286–292
Kawanishi N, Kato K, Takahashi M, Mizokam T, Otsuka Y, Imaizumi A, Shiva D, Yano H, Suzuki K (2013) Biochem Biophys Res Commun 441:573–578
Boroumand N, Samarghandian S, Hashemy SI (2018) J Herbmed Pharmacol 7:211–219
Chainani-Wu N (2003) J Altern Complement Med 9:161–168
Wright JS (2002) THEOCHEM 591:207–217
Jayaprakasha GK, Rao LJ, Sakariah KK (2006) Food Chem 98:720–724
Ruby AJ, Kuttan G, Dinesh Babu K, Rajasekharan KN, Kuttan R (1995) Cancer Lett 94:79–83
Llano S, Gómez S, Londoño J, Restrepo A (2019) Phys Chem. Chem Phys
Jitoe A, Masuda T, Tengah IGP, Suprapta DN, Gara IW, Nakatani N (1992) J Agric Food Chem 40:1337–1340
Sokmen M, Akram Khan M (2016) Inflammopharmacol 24:81–86
Sun JL, Ji HF, Shen L (2019) Food Nutr Res 63:3451
Mošovská S, Petáková P, Kaliňák M, Mikulajová A (2016) Acta Chim Slov 9:130–135
Shen L, Ji HF (2007) Spectrochim Acta, Part A 67:619–623
Rodrigues FC, Anilkumar NV, Thakur G (2019) Eur J Med Chem 177:76–104
Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB (2007) Molecular Pharmaceutics 4:807–818
Pari L, Tewas D, Eckel J (2008) Arch Physiol Biochem 114:127–149
Yallapu MM, Jaggi M, Chauhan SC (2012) Drug Discovery Today 17:71–80
Hao T, Wang K, Zhang S, Yang S, Wang P, Gao F, Zhao Y, Guo N, Yu P (2020) Eur J Med Chem 207:112798
Najafi M, Najafi M, Najafi H (2012) Can J Chem 90:915–926
Vo QV, Nam PC, Bay MV, Thong NM, Hieu LT, Mechler A (2019) RSC Adv 9:42020–42028
Santos JLF, Kauffmann AC, da Silva SC, Silva VCP, de Souza GLC (2020) J Mol Model 233
da S Filho AH, de Souza GLC (2020) Phys Chem. Chem Phys 22:17659–17667
Mendes RA, Almeida SKC, Soares IN, Barboza CA, Freitas RG, Brown A, GLC de Souza (2019) J Mol Model 89
Miar M, Shiroudi A , Pourshamsian K, Oliaey AR, Hatamjafari F (2020) J Chem Res 1–12
Muğlu H, Çavuş MS, Bakır T, Yakan H (2019) J Mol Struct 1196:819
Taguchi AT, O’Malley PJ, Wraight CA, Dikanov SA (2017) J Phys Chem 121:10199–10292
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, LiX Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, KleneM, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli JW, Ochterski C, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) GAUSSIAN 09 A
Feller D (1996) J Comput Chem 17:1571–1586
Becke AD (1993) J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1998) Phys Rev B 37:785–789
de Souza GLC, Peterson KA (2021) J Phys Chem A 125:198–208
Slabber CA, Grimmer CD, Robinson RS (2016) J Nat Prod 79:2726–2730
Siviero A, Gallo E, Maggini V, Gori L, Mugelli A, Firenzuoli F, Vannacci A (2015) J Herb Med 5:57–70
Modasiya MK, Patel VM (2012) Int J Pharm Life Sci 3:1490–1497
Jeliński T, Przybyłek M, Cysewski P (2019) Pharm Res 36:116
Cui Z, Yao L, Ye J, Wang Z, Hu Y (2021) J Mol Liq 338:116795
Savale SK (2017) Journal of PharmaSciTech 7:31–35
Ucisik MH, Küpcü S, Schuster B, Sleytr UB (2013) J Nanobiotechnol 11
Tomasi J, Mennucci B, Cammi R (2005) Chem Rev 105:2999–3093
Kolev TM, Velcheva EA, Stamboliyska BA, Spiteller M (2005) Int J Quantum Chem 102:1069–1079
You-Min S, Ruo-X W, Shi-Ling Y, Xian-Jie L, Cheng-Bu L (2004) Chin J Chem 22:827–830
Payton F, Sandusky P, Alworth WL (2007) J Nat Prod 70
Boulmokh Y, Belguidoum K, Meddour F, Amira-Guebailia H (2021) Struct Chem 32:1907–1923
Szeląg M, Urbaniak A, Bluyssen HAR (2015) Open Chem 13:17–31
Rimarcík J, Lukeš V, Klein E, Ilcin M (2010) THEOCHEM 952:25–30
Michalík M, Vagánek A, Poliak P (2014) Acta Chimica Slovaca 7:123–128
Xue Y, Zheng Y, An L, Dou Y, Liu Y (2014) Food Chem 151:198–206
Wang G, Xue Y, An L, Zheng Y, Dou Y, Zhang L, Liu Y (2015) Food Chem 171:89–97
Zheng YZ, Zhou Y, Liang Q, Chen DF, Guo R, Xiong CL, Xu XJ, Zhang ZN, Huang ZJ (2017) Dyes Pigm 141:179–187
Biela M, Rimarcík J, Senajova E, Kleinova A, Klein E (2020) Phytochemistry 180:112528
Lewars EG (2003) Kluwer Academic Publishers Norwell MA USA
Hatch FT, Lightstone FC, Colvein ME (2000) Environ Mol Mutagen 35:279–299
Schweizer J, Ressouche E (2001) Magnetism: molecules to materials I: models and experiments (chapter)
Savarese M, Bremond E, Ciofini I, Adamo C (2020) J Chem Theory Comput 16:3567–3577
Funding
No funding was received for conducting this study.
Author information
Authors and Affiliations
Contributions
Y. Boulmokh: Investigation, methodology, writing original draft, review and editing. K. Belguidoum; Investigation and review. F. Meddour; Investigation and review. H. Amira-Guebailia; Supervision, methodology, investigation, writing original draft, and review.
Corresponding author
Ethics declarations
Consent for publication
All authors whose names appear on the submission approved the version to be published.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Boulmokh, Y., Belguidoum, K., Meddour, F. et al. Enhanced antioxidant properties of novel curcumin derivatives: a comprehensive DFT computational study. Struct Chem 35, 825–839 (2024). https://doi.org/10.1007/s11224-023-02237-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02237-6