Abstract
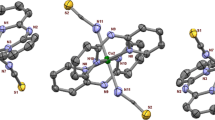
A dinuclear copper (II) complex, Cu2 (RCOO)4(L)2 (1) while [RCOO = benzoate, L = 2-amino-3-chloro-5-trifluoro methyl pyridine], with square pyramidal geometry with four carboxylate bridged groups has been reported. This dinuclear copper (II) unit (with the most robust, frequently occurring paddle wheel structure) was characterized by single crystal X-ray diffraction, IR, UV, CV, TGA, and EPR studies. It exhibits catechol oxidase activity in methanol medium and the catecholase activity was monitored by the UV–Vis spectroscopy. The kinetic parameters have been determined by using Michaelis–Menten equation which shows that the complex is an efficient catalyst with very high turnover number. Preliminary mechanistic investigation of the catalytic behavior was established with the help of ESI–MS spectra.
Graphical Abstract








Similar content being viewed by others
Data availability
The corresponding.CIF files are available in the Cambridge Structural Database (CSD); for complex 1, the CCDC number is 2,269,299.
Code availability
Not applicable.
References
Noro S, Kitagawa S, Kondo M, Seki K (2000) A new, methane adsorbent, porous coordination polymer [{CuSiF6 (4, 4′-bipyridine) 2} n]. Angew Chem Int Ed. https://doi.org/10.1002/1521-3773(20000616)39:12%3c2081::AID-ANIE2081%3e3.0.CO;2-A
Chatterjee A, Mondal P, Chakraborty P, Kumar B, Mondal S, Rizzoli C, Saha R, Adhikary B, Dey SK (2023) Strategic synthesis of heptacoordinated Fe (III) bifunctional complexes for efficient water electrolysis. Angew Chem Int Ed. https://doi.org/10.1002/anie.202307832
Liu GC, Li Y, Chi J, Xu N, Wang XL, Lin HY, Chen YQ (2020) Multi-functional fluorescent responses of cobalt complexes derived from functionalized amide-bridged ligand. Dyes Pigm. https://doi.org/10.1016/j.dyepig.2019
Han Z, Wang K, Chen Y, Li J, Teat SJ, Yang S, Cheng P (2022) A multicenter metal–organic framework for quantitative detection of multicomponent organic mixtures. CCS Chem. https://doi.org/10.31635/ccschem.022.202101642
Dai J-C, Wu X-T, Fu Z-Y, Cui C-P, Hu S-M, Du W-X, Wu L-M, Zhang H-H, Sun R-Q (2002) Synthesis, structure, and fluorescence of the novel cadmium (II)−trimesate coordination polymers with different coordination architectures. Inorg Chem. https://doi.org/10.1021/ic010794y
Han Z, Wang K, Zhou HC (2023) Preparation and quantitative analysis of multicenter luminescence materials for sensing function. Nat Protoc. https://doi.org/10.1038/s41596-023-00810-1
Dey SK, Bag B, Malik KMA, El FMS, Ribas J, Mitra S (2003) A quasi-tetrahedral Cu4 cluster and a helical-chain copper(II) complex with single syn−anti carboxylate bridges: crystal structure and magnetic properties. Inorg Chem. https://doi.org/10.1021/ic0206488
Reger DL, Debreczeni A, Reinecke B, Rassolov V, Smith MD, Semeniuc RF (2009) Highly organized structures and unusual magnetic properties of paddlewheel copper (II) carboxylate dimers containing the π− π stacking,1,8-naphthalimide synthon. Inorg Chem. https://doi.org/10.1021/ic901138h
Chattopadhyay K, Shaw BK, Saha SK, Ray D (2016) Unique trapping of paddlewheel copper (ii) carboxylate by ligand-bound Cu 2 fragments for [Cu 6] assembly. Dalton Trans. https://doi.org/10.1039/C6DT00103C
Viola MN, Ikram M, Rehman S, Akhtar M, AlDamen M, Schulzke C (2019) A paddle wheel dinuclear Copper(II) carboxylate: crystal structure, thermokinetic and magnetic properties. J Mol Struc. https://doi.org/10.1016/j.molstruc.2019.06.095
Jenniefer SJ, Muthiah PT (2013) Synthesis, characterization and X-ray structural studies of four copper (II) complexes containing dinuclear paddle wheel structures. Chem Cent J. https://doi.org/10.1186/1752-153X-7-35
Tonigold M, Volkmer D (2010) Comparative solvolytic stabilities of copper (II) nanoballs and dinuclear Cu (II) paddle wheel units. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2010.06.022
Walczak-Nowicka ŁJ, Herbet M (2022) Sodium benzoate-harmfulness and potential use in therapies for disorders related to the nervous system: a review. Nutrients. https://doi.org/10.3390/nu14071497
Agterberg FP, Provó Kluit HA, Driessen WL, Oevering H, Buijs W, Lakin MT, Spek AL, Reedijk J (1997) Dinuclear paddle-wheel copper (II) carboxylates in the catalytic oxidation of carboxylic acids. Unusual polymeric chains found in the single-crystal X-ray structures of [tetrakis(μ-1-phenylcyclopropane-1-carboxylato-O,O’)bis(ethanol-O)dicopper (II)] and catena-poly[[bis(μ-diphenylacetato-O:O’)dicopper](μ3-diphenylacetato-1-O:2-O’:1’-O’)(μ3-diphenylacetato-1-O:2-O’:2’-O’)]. Inorg Chem. https://doi.org/10.1021/ic9614733
Banu KS, Mukherjee M, Guha A, Bhattacharya S, Zangrando E, Das D (2012) Dinuclear copper(II) complexes: Solvent dependent catecholase activity. Polyhedron. https://doi.org/10.1016/j.poly.2012.06.087
Neves A, Rossi LM, Bortoluzzi AJ, Szpoganicz B, Wiezbicki C, Schwingel E, Haase W, Ostrovsky S (2002) Catecholase activity of a series of dicopper (II) complexes with variable Cu−OH (phenol) moieties. Inorg Chem. https://doi.org/10.1021/ic010708u
Rey NA, Neves A, Bortoluzzi AJ, Pich CT, Terenzi H (2007) Catalytic promiscuity in biomimetic systems: catecholase-like activity, phosphatase-like activity, and hydrolytic DNA cleavage promoted by a new dicopper (II) hydroxo-bridged complex. Inorg Chem. https://doi.org/10.1021/ic0613107
Osório RE, Peralta RA, Bortoluzzi AJ, de Almeida VR, Szpoganicz B, Fischer FL, Terenzi H, Mangrich AS, Mantovani KM, Ferreira DE (2012) Synthesis, magnetostructural correlation, and catalytic promiscuity of unsymmetric dinuclear copper (II) complexes: models for catechol oxidases and hydrolases. Inorg Chem. https://doi.org/10.1021/ic201876k
Banu KS, Chattopadhyay T, Banerjee A, Bhattacharya S, Suresh E, Nethaji M, Zangrando E, Das D (2008) Catechol oxidase activity of a series of new dinuclear copper (II) complexes with 3, 5-DTBC and TCC as substrates: syntheses, X-ray crystal structures, spectroscopic characterization of the adducts and kinetic studies. Inorg Chem. https://doi.org/10.1021/ic701332w
Mandal S, Mukherjee J, Lloret F, Mukherjee R (2012) Modeling tyrosinase and catecholase activity using new m-xylyl-based ligands with bidentate alkylamine terminal coordination. Inorg Chem. https://doi.org/10.1021/ic3013848
Torelli S, Belle C, Gautier-Luneau I, Pierre J, Saint-Aman E, Latour J, Le Pape L, Luneau D (2000) pH-Controlled change of the metal coordination in a dicopper (II) complex of the ligand H− BPMP: crystal structures, magnetic properties, and catecholase activity. Inorg Chem. https://doi.org/10.1021/ic991450z
Hwang IH, Jo YD, Kim H, Kim KB, Jung KD, Kim C, Kim Y, Kim SJ (2013) Catalytic transesterification reactions of one-dimensional coordination polymers containing paddle-wheel-type units connected by various bridging ligands. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2013.03.043
Meng Q, Chen C, Wang L (2016) Synthesis, structure and catalytic properties of a Cu(II) coordination polymer constructed from paddle-wheel building blocks. J Clust Sci. https://doi.org/10.1007/s10876-016-0994-y
Dutta B, Pal B, Dey S, Maity S, Ray PP, Mir MH (2022) Exploitation of structure-property relationships towards multi-dimensional applications of a paddle-wheel Cu(II) compound. Eur J Inorg Chem. https://doi.org/10.1002/ejic.202100904
Bruker SA, SAINT S, Bruker AXS Inc (2016) Madison, Wisconsin
Sheldrick GM (2015) Integrated apace-group and crystal structure determination. Acta Cryst A 71:3–8
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C 71:3–8
Dey SK, Shit S, Mitra S, Thompson LK, Malik KMA (2007) An antiferromagnetically coupled trinuclear Cu (II) complex containing μ (O, O′), μ (O) carboxylates and μ (O) phenoxide bridges. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2006.09.030
Ackermann MN (1971) Infrared spectra of inorganic and coordination compounds (Nakamoto. ACS Publications, Kazuo)
Dey SK, Bag B, Zhou Z, Chan AS, Mitra S (2004) Synthesis, characterization and crystal structure of a monomeric and a macrocyclic copper (II) complex with a large cavity using benzylacetylacetone ligand. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2003.12.014
Chatterjee A, Chakraborty P, Kumar B, Rizzoli C, Mandal P, Dey SK (2022) Synthesis, characterization of one Cr (III) complex: an efficient chemosensor for Cr (III) ions and designing of a molecular logic gate. J Mol Struct. https://doi.org/10.1016/j.molstruc.2022.132486
Sarkar M, Clerac R, Mathoniere C, Hearns NG, Bertolasi V, Ray D (2010) New μ4-oxido-bridged copper benzoate quasi-tetrahedron and bis-μ3-hydroxido-bridged copper azide and copper thiocyanate stepped cubanes: core conversion, structural diversity, and magnetic properties. Inorg Chem. https://doi.org/10.1021/ic100356y
Ojwach SO, Okemwa TT, Attandoh NW, Omondi B (2013) Structural and kinetic studies of the polymerization reactions of ε-caprolactone catalyzed by (pyrazol-1-ylmethyl)pyridine Cu(ii) and Zn(ii) complexes. Dalton Trans. https://doi.org/10.1039/C3DT51338F
Zippel F, Ahlers F, Werner R, Haase W, Nolting H-F, Krebs B (1996) Structural and functional models for the dinuclear copper active site in catechol oxidases: syntheses, X-ray crystal structures, magnetic and spectral Properties, and X-ray absorption spectroscopic studies in solid state and in solution. Inorg Chem. https://doi.org/10.1021/ic9513604
Jana A, Aliaga-Alcalde N, Ruiz E, Mohanta S (2013) Structures, magnetochemistry, spectroscopy, theoretical study, and catechol oxidase activity of dinuclear and dimer-of-dinuclear mixed-valence MnIIIMnII complexes derived from a macrocyclic ligand. Inorg Chem. https://doi.org/10.1021/ic400916h
Das M, Nasani R, Saha M, Mobin SM, Mukhopadhyay S (2015) Nickel (II) complexes with a flexible piperazinyl moiety: studies on DNA and protein binding and catecholase like properties. Dalton Trans. https://doi.org/10.1039/C4DT02675F
Vogel AI (1961) A text book of quantitative inorganic analysis. Wiley, New York
Mukherjee D, Nag P, Shteinman AA, Vennapusa SR, Mandal U, Mitra M (2021) Catechol oxidation promoted by bridging phenoxo moieties in a bis (μ-phenoxo)-bridged dicopper (ii) complex. RSC Adv. https://doi.org/10.1039/D1RA02787E
Adhikari S, Banerjee A, Nandi S, Fondo M, Sanmartín-Matalobos J, Das D (2015) Structure, magnetism and catecholase activity of the first dicopper (ii) complex having a single μ-alkoxo bridge. RSC Adv. https://doi.org/10.1021/acs.jpcc.9b09272
Yang C-T, Vetrichelvan M, Yang X, Moubaraki B, Murray KS, Vittal JJ (2004) Syntheses, structural properties and catecholase activity of copper (II) complexes with reduced Schiff base N-(2-hydroxybenzyl)-amino acids. Dalton Trans. https://doi.org/10.1039/B310262A
Sreenivasulu B, Zhao F, Gao S, Vittal JJ (2006) Synthesis, structures and catecholase activity of a new series of dicopper (II) complexes of reduced Schiff base ligands. Eur J Inorg Chem. https://doi.org/10.1002/ejic.200600022
Caglar S, Aydemir IE, Adıgüzel E, Caglar B, Demir S, Buyukgungor O (2013) Four copper (II) diclofenac complexes with pyridine derivatives: synthesis, crystal structures, spectroscopic properties, thermal analysis and catechol oxidase activities. Inorg Chim Acta. https://doi.org/10.1016/j.ica.2013.09.013
Acknowledgements
SKD gratefully acknowledge the Higher Education department, West Bengal, for funding the instrumental facilities to the SKBU. We are gratefully thankful to Dr. Rajat Saha of Kazi Nazrul University, WB, India, for his assistance.
Funding
This work was financially supported by the WB-DST (Project Memo No 746 (Sanc)/ST/P. S&T/15G dt. 22.11.2016.). We acknowledge the WB-DST fellowship (partial) and SVMM fellowship (partial) to P. C. and the INSPIER fellowship to A.C. (Ref. No. DST/INSPIRE Fellowship/2017/IF170767 dt. 04.07.2018) was funded by DST, New Delhi.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. PC and AC contributed equally. Complex preparation, data collection, and analysis were performed by Priyanka Chakraborty and Abhishikta Chatterjee. The first draft of the manuscript was written by Priyanka Chakraborty and Abhishikta Chatterjee and all authors commented on draft versions of the manuscript. Methodology, formal analysis, and visualization were performed by Priyanka Chakraborty and Abhishikta Chatterjee. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chakraborty, P., Chatterjee, A., Mandal, R. et al. Synthesis, crystal structure, and catechol oxidase activity of a di-nuclear paddle-wheel Cu (II) carboxylate complex. Struct Chem 35, 791–799 (2024). https://doi.org/10.1007/s11224-023-02225-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02225-w