Abstract
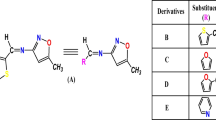
Many organic chromophores possess excellent nonlinear optical (NLO) properties. In spite of this, the performance of these chromophores in second-order nonlinear optical applications is limited due to the high symmetry and short dipole moment of the parent molecules, which can lead to a weak response or high susceptibility to molecular orientation dependency. To address this challenge, we designed a series of new [1,2,4] triazolo[3,4-b] [1,3,4] thiadiazole derivative chromophores C1–C7 and studied their second-order NLO property through density functional theory (DFT) by substitution of different donor functional groups. To this end, several hybrid functionals such as B3LYP/6–311 + + G (d, p), CAM B3LYP/6–311 + + G (d, p), and ωB97XD/6–311 + + G (d, p) were employed to carry out quantum chemical calculations, which included calculations and evaluations of frequency-dependent dipole moment, linear polarizability, and first hyperpolarizability values. In addition, natural bond orbital (NBO) analysis, intramolecular charge transfer (ICT) mechanism, electronic charge density analysis, highest occupied molecular orbital (HOMO), lowest unoccupied molecular orbital (LUMO), and vertical energy transitions are studied with time-independent and time-dependent level density functional theory. The results demonstrate that C7 is the most efficient chromophore among the other chromophores investigated, with a significant first hyperpolarizability value of 105,032.98 × 10−34. This study provides insights into how to design second-order NLO active organic chromophores with better performance for optoelectronic applications.







Similar content being viewed by others
Availability of data and materials
All data are available on request to the corresponding author.
Code availability
Not applicable.
References
Marder SR, Perry JW (1993) Molecular materials for second-order nonlinear optical applications. Adv Mater 5:804–815. https://doi.org/10.1002/adma.19930051104
Abdullah M, Bakhtiar H, Krishnan G, Aziz M, Danial W, Islam S (2019) Transition from saturable absorption to reverse saturable absorption of carmoisine dye under low-powered continuous wave laser excitation. Opt Laser Technol 115:97–103. https://doi.org/10.1016/j.optlastec.2019.01.032
Bredas JL, Adant C, Tackx P, Persoons A, Pierce B (1994) Third-order nonlinear optical response in organic materials: theoretical and experimental aspects. Chem Rev 94:243–278. https://doi.org/10.1021/cr00025a008
Notake T, Takeda M, Okada S, Hosobata T, Yamagata Y, Minamide H (2019) Characterization of all second-order nonlinear-optical coefficients of organic N-benzyl-2-methyl-4-nitroaniline crystal. Sci Rep 9:1–8. https://doi.org/10.1038/s41598-019-50951-1
Najare MS, Patil MK, Garbhagudi M, Yaseen M, Inamdar SR, Khazi IAM (2021) Design, synthesis and characterization of π-conjugated 2, 5-diphenylsubstituted-1, 3, 4-oxadiazole-based D-π-A-π’-D′ form of efficient deep blue functional materials: Photophysical properties and fluorescence “Turn-off” chemsensors approach. J Mol Liq 328:11544. https://doi.org/10.1016/j.molliq.2021.115443
Ullah F, Deng N, Qiu F (2021) Recent progress in electro-optic polymer for ultra-fast communication. PhotoniX 2:13. https://doi.org/10.1186/s43074-021-00036-y
Abudurusuli A, Li J, Pan S (2021) A review on the recently developed promising infrared nonlinear optical materials. Dalton Trans 50:3155–3160. https://doi.org/10.1039/d1dt00054c
Tsutsumi N, Morishima M, Sakai W (1998) Nonlinear optical (NLO) polymers. 3. NLO polyimide with dipole moments aligned transverse to the imide linkage. Macromolecules 31:7764–7769. https://doi.org/10.1021/ma9803436
Breitung EM, Shu C-F, McMahon RJ (2000) Thiazole and thiophene analogues of donor− acceptor stilbenes: molecular hyperpolarizabilities and structure− property relationships. J Am Chem Soc 122:1154–1160. https://doi.org/10.1021/ja9930364
Colombo A, Dragonetti C, Guerchais V, Hierlinger C, Zysman-Colman E, Roberto D (2020) A trip in the nonlinear optical properties of iridium complexes. Coord Chem Rev
Prasad PN (1989) Nonlinear optical effects in organic molecules and polymers-theory, measurements and devices. In Optical Materials Technology for Energy Efficiency and Solar Energy Conversion. Proc SPIE Int Soc Opt Photonics 1016:2–11. https://doi.org/10.1117/12.949907
Dalton LR, Sullivan PA, Bale DH (2010) Electric field poled organic electro-optic materials: state of the art and future prospects. Chem Rev 110:25–55. https://doi.org/10.1021/cr9000429
Luo X, Li Z, Guo Y, Yao J, Wu Y (2019) Recent progress on new infrared nonlinear optical materials with application prospect. J Solid State Chem 270:674–687. https://doi.org/10.1016/j.jssc.2018.12.036
You J, Bongu S, Bao Q, Panoiu N (2019) Nonlinear optical properties and applications of 2D materials: theoretical and experimental aspects. Nanophotonics 8:63–97. https://doi.org/10.1515/nanoph-2018-0106
Karna SP (2000) Electronic and nonlinear optical materials: the role of theory and modeling. J Phys Chem A 104:4671–4673. https://doi.org/10.1021/jp001296y
Bhattacharya S, Biswas C, Raavi SS, Venkata Suman Krishna J, Vamsi Krishna N, Giribabu L, Soma VR (2019) Synthesis, optical, electrochemical, DFT studies, NLO properties, and ultrafast excited state dynamics of carbazole-induced phthalocyanine derivatives. J Phys Chem C 123:11118–11133. https://doi.org/10.1021/acs.jpcc.9b01531
Akram M, Adeel M, Khalid M, Tahir MN, Khan MU, Asghar MA, Ullah MA, Iqbal M (2018) A combined experimental and computational study of 3-bromo-5-(2, 5-difluorophenyl) pyridine and 3, 5-bis (naphthalen-1-yl) pyridine: insight into the synthesis, spectroscopic, single crystal XRD, electronic, nonlinear optical and biological properties. J Mol Struct 1160:129–141. https://doi.org/10.1016/j.molstruc.2018.01.100
Ahmad MS, Khalid M, Shaheen MA, Tahir MN, Khan MU, Braga AA, Shad HA (2018) Synthesis and XRD, FT-IR vibrational, UV–vis, and nonlinear optical exploration of novel tetra substituted imidazole derivatives: a synergistic experimental-computational analysis. J Phys Chem Solids 115:265–276. https://doi.org/10.1016/j.jpcs.2017.12.054
Shen Y, Tang W, Lin X (2022) Advances in second-order nonlinear optical sulfates. Coord Chem Rev 459:214443. https://doi.org/10.1016/j.ccr.2022.214443
Wu J, Luo J, Jen AK-Y (2020) High-performance organic second-and third-order nonlinear optical materials for ultrafast information processing. J Mat Chem C 8:15009–15026. https://doi.org/10.1039/D0TC03224G
Yahya M, Nural Y, Seferoğlu Z (2022) Recent advances in the nonlinear optical (NLO) properties of phthalocyanines: a review. Dyes and Pigm 198:109960. https://doi.org/10.1016/j.dyepig.2021.109960
Bosshard C, Sutter K, Prêtre P, Hulliger J, Flörsheimer M, Kaatz P, Günter P (1995) Organic nonlinear optical materials. CRC Press, London. https://doi.org/10.1201/9780429114090
Suresh S, Ramanand A, Jayaraman D, Mani P (2012) Review on theoretical aspect of nonlinear optics. Rev Adv Mater Sci 30:175–183
Marder SR (2006) Organic nonlinear optical materials: where we have been and where we are going. Chem Commun 131–134. https://doi.org/10.1039/B512646K
Laurent AD, Adamo C, Jacquemin D (2014) Dye chemistry with time-dependent density functional theory. Phys Chem Chem Phys 16:14334–14356. https://doi.org/10.1039/C3CP55336A
Laurent AD, Jacquemin D (2013) TD-DFT benchmarks: a review. Int J Quantum Chem 113:2019–2039. https://doi.org/10.1002/qua.24438
Wu S, Shi J, Chen J, Hu D, Zang L, Song B (2021) Synthesis, antibacterial activity, and mechanisms of novel 6-sulfonyl-1, 2, 4-triazolo [3, 4-b][1, 3, 4] thiadiazole derivatives. J Agric Food Chem 69:4645–4654. https://doi.org/10.1021/acs.jafc.1c01204
Radwan AA, Alanazi FK, Al-Agamy MH (2017) 1, 3, 4-Thiadiazole and 1, 2, 4-triazole-3 (4H)-thione bearing salicylate moiety: synthesis and evaluation as anti-Candida albicans. Braz J Pharm Sci 53:e15239. https://doi.org/10.1590/s2175-97902017000115239
Lin L, Liu H, Wang DJ, Hu YJ, Wei XH (2017) Synthesis and biological activities of 3, 6-disubstituted-1, 2, 4-triazolo-1, 3, 4-thiadiazole derivatives. Bull Chem Soc of Ethiop 31:481–489. https://doi.org/10.4314/bcse.v31i3.12
Pandey AK, Shukla DV, Singh V, Narayan V (2018) Structural, IR spectra NBO, TDDFT, AIM calculation, biological activity and docking property of [1, 2, 4]-triazolo [3, 4-b][1, 3, 4] thiadiazole. Egypt J Basic Appl Sci 5:280–288. https://doi.org/10.1016/j.ejbas.2018.10.001
Gao F, Wang T, Xiao J, Huang G (2019) Antibacterial activity study of 1, 2, 4-triazole derivatives. Eur J Med Chem 173:274–281. https://doi.org/10.1016/j.ejmech.2019.04.043
Shamroukh AH, Hegab MI (2020) A review on synthesis, therapeutic, and computational studies of substituted 1, 3, 4 thiadiazole derivatives. Egyp J Chem 63:4387–4408. https://doi.org/10.21608/ejchem.2020.25343.2492
Bhat KS, Prasad DJ, Poojary B, Holla BS (2004) Synthesis of some new 1, 2, 4-triazolo [3, 4-b]-thiadiazole derivatives as possible anticancer agents. Phosphorus Sulfur Silicon Relat Elem 179:1595–1603. https://doi.org/10.1080/10426500490464186
Fusco S, Centore R, Riccio P, Quatela A, Stracci G, Archetti G, Kuball H-G (2008) NLO-active polymers containing triazolo-thiadiazole segments. Polymer 49:186–191. https://doi.org/10.1016/j.polymer.2007.11.016
Capobianco A, Centore R, Fusco S, Peluso A (2013) Electro-optical properties from CC2 Calculations: a comparison between theoretical and experimental results. Chem Phys Lett 580:126–129. https://doi.org/10.1016/j.cplett.2013.07.004
Doddamani RV, Rachipudi PS, Inamdar SR, Kariduraganavar MY (2019) Enhancement of nonlinear optical and thermal properties of polyurethanes by modifying the chromophores with fused heterocyclic and pyrimidine rings. Polym Eng Sci 59:500–509. https://doi.org/10.1002/pen.24957
Me F, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Petersson G, Nakatsuji H (2016) Gaussian 16. Gaussian, Inc., Wallingford, CT
Zhang IY, Wu J, Xu X (2010) Extending the reliability and applicability of B3LYP. Chem Commun 46:3057–3070. https://doi.org/10.1039/C000677G
Dreuw A, Head-Gordon M (2005) Single-reference ab initio methods for the calculation of excited states of large molecules. Chem Rev 105:4009–4037. https://doi.org/10.1021/cr0505627
Parr RG, Szentpály LV, Liu S (1999) Electrophilicity index. J Am Chem Soc 121:1922–1924. https://doi.org/10.1021/ja983494x
Mehboob MY, Hussain R, Adnan M, Farwa U, Irshad Z, Janjua MR (2021) Theoretical modelling of novel indandione-based donor molecules for organic solar cell applications. J Phy Chem Solids 162:110508. https://doi.org/10.1016/j.jpcs.2021.110508
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phy 10:6615–6620. https://doi.org/10.1039/B810189B
Pandey N, Mehata MS, Pant S, Tewari N (2021) Structural, electronic and NLO properties of 6-aminoquinoline: a DFT/TD-DFT study. J Fluoresc 31:1719–1729. https://doi.org/10.1007/s10895-021-02788-z
Oudar JD (1977) Optical nonlinearities of conjugated molecules. Stilbene derivatives and highly polar aromatic compounds. J Chem Phys 67:446–457. https://doi.org/10.1063/1.434888
Zyss J (1979) Hyperpolarizabilities of substituted conjugated molecules. II. Substituent effects and respective σ–π contributions. J Chem Phys 70:3341–3349. https://doi.org/10.1063/1.437919
Oudar JL, Chemla DS (1977) Hyperpolarizabilities of the nitroanilines and their relations to the excited state dipole moment. J Chem Phys 66:2664–2668. https://doi.org/10.1063/1.434213
Jiang X, Zhao S, Lin Z, Luo J, Bristowe PD, Guan X, Chen C (2014) The role of dipole moment in determining the nonlinear optical behavior of materials: ab initio studies on quaternary molybdenum tellurite crystals. J Mater Chem C 2:530–537. https://doi.org/10.1039/C3TC31872A
Yanagi K, Kobayashi T, Hashimoto H (2003) Origin of transition dipole-moment polarizability and hyperpolarizability in hydrazones. Phys Rev B 67:115122. https://doi.org/10.1103/PhysRevB.67.115122
Zhang T, Brumboiu IE, Grazioli C, Guarnaccio A, Coreno M, de Simone M, Santagata A, Rensmo H, Brena B, Lanzilotto V, Puglia C (2018) Lone-Pair delocalization effects within electron donor molecules: the case of triphenylamine and its thiophene-analog. J Phys Chem C 122:17706–17717. https://doi.org/10.1021/acs.jpcc.8b06475
Parthasarathy V, Pandey R, Das PK, Castet F, Blanchard-Desce M (2018) Linear and nonlinear optical properties of tricyanopropylidene-based merocyanine dyes: synergistic experimental and theoretical investigations. ChemPhysChem 19:187–197. https://doi.org/10.1002/cphc.201701143
Drissi M, Benhalima N, Megrouss Y, Rachida R, Chouaih A, Hamzaoui F (2015) Theoretical and experimental electrostatic potential around the m-nitrophenol molecule. Molecules 20:4042–4054. https://doi.org/10.3390/molecules20034042
Sebastian S, Sundaraganesan N (2010) The spectroscopic (FT-IR, FT-IR gas phase, FT-Raman and UV) and NBO analysis of 4-Hydroxypiperidine by density functional method. Spectrochim Acta Part A Mol Biomol Spectrosc 75:941–952. https://doi.org/10.1016/j.saa.2009.11.030
Xavier S, Periandy S, Ramalingam S (2015) NBO, conformational, NLO, HOMO–LUMO, NMR and electronic spectral study on 1-phenyl-1-propanol by quantum computational methods. Spectrochim Acta Part A Mol Biomol Spectrosc 137:306–320. https://doi.org/10.1016/j.saa.2014.08.039
Zarate X, Schott E, Gomez T, Arratia-Pérez R (2013) Theoretical study of sensitizer candidates for dye-sensitized solar cells: peripheral substituted dizinc pyrazinoporphyrazine–phthalocyanine complexes. J Phys Chem A 117:430–438. https://doi.org/10.1021/jp3067316
Srivastava A, Rawat P, Tandon P, Singh RN (2012) A computational study on conformational geometries, chemical reactivity and inhibitor property of an alkaloid bicuculline with γ-aminobutyric acid (GABA) by DFT. Comput Theor Chem 993:80–89. https://doi.org/10.1016/j.comptc.2012.05.025
Landis CR, Cleveland T, Firman TK (1998) Valence bond concepts applied to the molecular mechanics description of molecular shapes. 3. Applications to transition metal alkyls and hydrides. J Am Chem Soc 120:2641–2649. https://doi.org/10.1021/ja9734859
Reed AE, Weinstock RB, Weinhold F (1985) Natural population analysis. J Chem Phys 83:735–746. https://doi.org/10.1063/1.449486
Ayare NN, Rajeshirke M, Sreenath MC, Chitrambalam S, Joe IH, Sekar N (2019) NLO-phoric emissive quinoxaline analog of Quinoline Yellow 54 and Z-Scan studies. ChemistrySelect 4:3752–3761. https://doi.org/10.1002/slct.201900040
Acknowledgements
The authors gratefully thank the Chairman, Department of PG studies in Chemistry and Coordinator of Molecular Modelling Lab under UPE FAR-I & DST PURSE Phase-II Programme at Karnatak University Dharwad, for providing the computational facility to the present work.
Author information
Authors and Affiliations
Contributions
The conceptualization and design of the study were collective efforts from all of the authors. Mr. Balachandar Waddar was responsible for the material simulations, data gathering, and analysis, while Dr. Saidi Reddy Parne was responsible for the conceptualization, reviewing and editing, resources, and supervision of the project. Dr. Suman Gandi, Dr. Gurusiddappa R. Prasanth, Mr. Mohammed Yaseen, and Dr. Mahadevappa Y. Kariduraganavar were responsible for the reviewing and editing, as well as the resources and formal analysis. Previous versions of the paper were discussed and commented on by all of the authors. The final text was reviewed and approved by all of the authors.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Waddar, B., Parne, S.R., Gandi, S. et al. The second-order nonlinear optical properties of novel triazolo[3,4-b] [1, 3, 4] thiadiazole derivative chromophores using DFT calculations. Struct Chem 35, 253–264 (2024). https://doi.org/10.1007/s11224-023-02178-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02178-0