Abstract
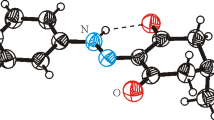
The current research presents simple synthesis, single crystal X-ray structure determination, Hirshfeld surface (HS) analysis, crystal voids studies, and density function theory (DFT) calculations of N-carbamothioylbenzamide and 1,3,5-triazinane-2,4,6-trithione co-crystal. Consequently, single crystal X-ray analysis revealed that the synthesized compounds are co-crystallized in a monoclinic crystal system with space group of P21/c and Z = 4. HS analysis visualized, explored, and subsequently quantified intermolecular interactions present in the crystal lattices of the co-crystallized compounds. HS analysis indicated close contacts associated with molecular interactions in greater depth and augments the significance of hydrogen atom contacts in crystal packing. Similarly, void studies assessed mechanical stability, and indicated the absence of any large cavity within the packed co-crystal. Moreover, the optimized molecular structures, using DFT at B3LYP/6–311G(d,p) level, were compared with the experimentally determined ones. HOMO–LUMO energy gap was determined and the frequencies as well as the molecular electrostatic potential surface were calculated at the B3LYP/6–311 G level. The DFT computed geometry was found to be in good agreement with the crystal data geometry.














Similar content being viewed by others
Availability of data and materials
The data is available with the manuscript.
References
Rigane I, Walha S, Salah AB (2016) Hydrogen bonding in thiobenzamide synthon and its Cadmium complex: Crystal structure and Hirshfeld analysis. J Chem Sci 128(9):1395–1404. https://doi.org/10.1007/s12039-016-1133-x
Papa M, Chiarotto I, Feroci M (2017) Willgerodt-Kindler reaction of benzaldehydes: a comparative study for a sustainable synthesis of secondary thiobenzamides. Chem Sel 2(10):3207–3210. https://doi.org/10.1002/slct.201700507
Hou RS, Wang HM, Tsai HH, Chen LC (2006) Synthesis of 2-phenylthiazoles from -tosyloxyketones and thiobenzamide in [Bmim][PF6] ıonic liquid at ambient temperature. J Chinese Chem Soc 53:863–866
Yu KL, Torri AF, Luo G, Cianci C, Young KG, Danetz S, Tiley L, Krystal M, Meanwell NA (2002) Structure activity relationships for a series of thiobenzamide influenza fusion inhibitors derived from 1,3,3-trimethyl-5-hydroxy-cyclohexylmethylamine. Bioorg Med Chem Lett 12(23):3379–3382. https://doi.org/10.1016/S0960-894X(02)00761-8
Long TR, Wongrakpanich A, Do AV, Salem AK, Bowden NB (2015) Long-term release of a thiobenzamide from a backbone functionalized poly (lactic acid). Pol Chem 6(40):7188–7195. https://doi.org/10.1039/c5py01059d
Jia Y, Zhang Y, Huang Y, Chen L, Wang M, Zhang Y (2021) Synthesis of trimethylacetyl thiobenzamide and its flotation separation performance of galena from sphalerite. App Surf Sci 569:151055. https://doi.org/10.1016/j.apsusc.2021.151055
Özcan M, Dehri I (2004) Electrochemical and quantum chemical studies of some sulphur-containing organic compounds as inhibitors for the acid corrosion of mild steel. Prog Org Coat 51(3):181–187. https://doi.org/10.1016/j.porgcoat.2004.07.017
Pandarinathan V, Lepková K, Bailey SI, Gubner R (2013) Inhibition of under-deposit corrosion of carbon steel by thiobenzamide. J Electrochem Soc 160(9):C432. https://doi.org/10.1149/2.078309jes
Akrivos PD (2001) Recent studies in the coordination chemistry of heterocyclic thiones and thionates. Coord Chem Rev 213(1):181–210. https://doi.org/10.1016/S0010-8545(00)00372-6
Henke KR, Hutchison AR, Krepps MK, Parkin S, Atwood DA (2001) Chemistry of 2,4,6-trimercapto-1,3,5-triazine (TMT): acid dissociation constants and group 2 complexes. Inorg Chem 40(17):4443–4447. https://doi.org/10.1021/ic0103188
Lobana TS (2021) Heterocyclic-2-thione derivatives of group 10–12 metals: Coordination versatility, activation of CS (thione) bonds and biochemical potential. Coord Chem Rev 441:213884. https://doi.org/10.1016/j.ccr.2021.213884
Bailey JR, Hatfield MJ, Henke KR, Krepps MK, Morris JL, Otieno T, Simonetti KD, Wall EA, Atwood DA (2021) Transition metal complexes of 2,4,6-trimercapto-1,3,5-triazine (TMT): potential precursors to nanoparticulate metal sulfides. J Organomet Chem 623(1–2):185–190. https://doi.org/10.1016/S0022-328X(00)00740-3
He K, Zhou H, Zhang Y, Tang X, Luo X, Han H (2021) Enhancing flotation of smithsonite by using 1,3,5-triazinane-2,4,6-trithione as sulfidation. Physicochem Probl Miner Process 57(5):1–14. https://doi.org/10.37190/ppmp/139903
Cherukuvada S, Kaur R, Row TNG (2016) Co-crystallization and small molecule crystal form diversity: from pharmaceutical to materials applications. Cryst Eng Comm 18(44):8528–8555. https://doi.org/10.1039/C6CE01835A
Rodrigues M, Baptista B, Lopez JA, Sarraguça MC (2018) Pharmaceutical cocrystallization techniques. Advances and challenges. Int J Pharm 547(1–2):404–420. https://doi.org/10.1016/j.ijpharm.2018.06.024
Aziz H, Saeed A, Khan MA, Afridi S, Jabeen F, Hashim M (2020) Novel N-acyl-1H-imidazole-1-carbothioamides: Design, synthesis, biological and computational studies. Chem Biodivers 17(3):e1900509. https://doi.org/10.1002/cbdv.201900509
Aziz H, Saeed A, Khan MA, Afridi S, Jabeen F (2021) Synthesis, characterization, antimicrobial, anti-oxidant and computational evaluation of N-acyl-morpholine-4-carbothioamides. Mol Divers 25:763–776. https://doi.org/10.1007/s11030-020-10054-w
Hu J, Zhang X (2017) Catalytic selectivity, and process optimization of the trimerization of toluene diisocyanate. J Mater Sci 52(20):12524–12539. https://doi.org/10.1007/s10853-017-1372-3
Golling FE, Pires R, Hecking A, Weikard J, Richter F, Danielmeier K, Dijkstra D (2019) Polyurethanes for coatings and adhesives-chemistry and applications. Polym Int 68(5):848–855. https://doi.org/10.1002/pi.5665
CrysAlisPro, Agilent Technologies Ltd, Yarnton, Oxfordshire, England (2018)
Sheldrick GM (2015) SHELXT-Integrated space group and crystal structure determination. Acta Cryst A71:3–8. https://doi.org/10.1107/S2053273314026370
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst C71:3–8. https://doi.org/10.1107/S2053229614024218
Farrugia LJ (2012) WinGX and ORTEP for Windows: an update. J Appl Cryst 45:849–854. https://doi.org/10.1107/S0021889812029111
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Monge LR, Taylor R, van de Streek J, Wood PA (2008) Mercury CSD 2.0-New features for the visualization and investigation of crystal structures. J Appl Cryst 41:466–470. https://doi.org/10.1107/S0021889807067908
Hirshfeld FL (1977) Bonded-atom fragments for describing molecular charge densities. Theor Chim Acta 44:129–138. https://doi.org/10.1007/BF00549096
Spackman MA, Jayatilaka D (2009) Hirshfeld surface analysis Cryst Eng Comm 11(1):19–32. https://doi.org/10.1039/B818330A
Turner MJ, McKinnon JJ, Wolff SK, Grimwood DJ, Spackman PR, Jayatilaka D, Spackman MA (2017) CrystalExplorer17. The University of Western Australia
Rigaku OD (2018) CrysAlis PRO Rigaku Oxford Diffraction, Yarnton, England
Guo F, Cheung EY, Harris KDM, Pedireddi VR (2006) Contrasting solid-state structures of trithiocyanuric acid and cyanuric acid. Cryst Growth Des 6(4):846–848. https://doi.org/10.1021/cg0601094
Brito I, Albanez J, Bolte M (2010) Trithiacyanuric acid: a second triclinic polymorph. Acta Crystallogr Sect E Struct E 66(9):o2382–o2383. https://doi.org/10.1107/S1600536810033234
Nagarajan V, Pedireddi VR (2014) Preparation of multiple cocrystals of trithiocyanuric acid with some N-donor compounds. Cryst Growth Des 14(9):4803–4810. https://doi.org/10.1021/cg500961n
Venkatesan P, Thamotharan S, Ilangovan A, Liang H, Sundius T (2016) Crystal structure, hirshfeld surfaces and DFT computation of NLO active (2E)-2-(ethoxycarbonyl)-3-[(1-methoxy-1-oxo-3-phenylpropan-2-yl)amino]prop-2-enoic acid. Spectrochim Acta Part A 153:625–636. https://doi.org/10.1016/j.saa.2015.09.002
McKinnon JJ, Jayatilaka D, Spackman MA (2007) Towards quantitative analysis of intermolecular interactions with Hirshfeld surfaces. Chem Commun 37:3814–3816. https://doi.org/10.1039/B704980C
Hartwar VR, Sist M, Jorgensen MRV, Mamakhel AH, Wang X, Hoffmann CF, Sugimoto K, Overgaard J, Iversen BB (2015) Quantitative analysis of intermolecular interactions in orthorhombic rubrene. IUCrJ 2(5):563–574. https://doi.org/10.1107/S2052252515012130
Turner MJ, McKinnon JJ, Jayatilaka D, Spackman MA (2011) Visualisation, and characterization of voids in crystalline materials. Cryst Eng Comm 13(6):1804–1813. https://doi.org/10.1039/C0CE00683A
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Vreven TJ, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin N, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli CJ, Ochterski W, Martin LR, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian Inc., (Wallingford, CT)
Acknowledgements
Hamid Aziz is highly grateful to the Higher Education Commission (HEC), Pakistan for providing indigenous scholarship. We also thank the University of Otago for purchase of the diffractometer and the Chemistry Department, University of Otago for support of the work of Jim Simpson. Tuncer Hokelek is grateful to Hacettepe University Scientific Research Project Unit (Grant No. 013 D04 602 004).
Funding
Hamid Aziz is highly grateful to the Higher Education Commission (HEC), Pakistan for providing indigenous scholarship. We also thank the University of Otago for purchase of the diffractometer and the Chemistry Department, University of Otago for support of the work of Jim Simpson. Tuncer Hokelek is grateful to Hacettepe University Scientific Research Project Unit (Grant No. 013 D04 602 004).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• N-Carbamothioylbenzamide and 1,3,5-triazinane-2,4,6-trithione were co-crystallized.
• X-ray determined monoclinic crystal system and P21/c space group of the co-crystal.
• Hirshfeld surface analysis revealed hydrogen atom contacts as crucial for crystal packing.
• Void analysis suggests stability and absence of any large cavity within the co-crystal.
• Density functional theory computed geometry was aligned with crystal data geometry.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Aziz, H., Saeed, A., Simpson, J. et al. Synthesis, single crystal X-ray structure determination, Hirshfeld surface analysis, crystal voids studies, and density functional theory calculations of N-carbamothioylbenzamide and 1,3,5-triazinane-2,4,6-trithione co-crystal. Struct Chem 35, 305–319 (2024). https://doi.org/10.1007/s11224-023-02171-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02171-7