Abstract
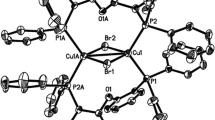
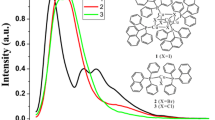
In this work, we report the temperature-dependent solid-state photoluminescence properties of the phosphine-supported copper(I) − halide dinuclear complexes, [(bdpb)CuX]2 (bdpb = 1,4-bis(diphenyl-phosphaneyl)butane, X = I, 1; Br, 2; Cl, 3). The X-ray diffraction analyses reveal that the metal centers exhibit a distorted tetrahedral structure in three complexes, wherein the dinuclear centers are separated via two bridging halide anions with the long Cu‒Cu distances [3.109(2) Å for 1, 3.200(2) Å for 2 and 3.181(2) Å for 3] in the X-ray structure. The luminescence properties of the three compounds were investigated in detail as a function of the temperature showing reversible luminescence thermochromism with an intense blue emission at low temperature.






Similar content being viewed by others
Availability of data and materials
Traditional publishing route.
References
Tian J, Jiang F, Yuan D et al (2020) Electric-field assisted in situ hydrolysis of bulk metal–organic frameworks (MOFs) into ultrathin metal oxyhydroxide nanosheets for efficient oxygen evolution. Angew Chem Int Ed 59:13101–13108. https://doi.org/10.1002/anie.202004420
Ma Y-J, Hu J-X, Han S-D et al (2020) Manipulating on/off single-molecule magnet behavior in a dy(III)-based photochromic complex. J Am Chem Soc 142:2682–2689. https://doi.org/10.1021/jacs.9b13461
Jiang X, Han S, Wang A et al (2021) The tri(imidazole)‐derivative moiety: a new category of electron acceptors for the design of crystalline hybrid photochromic materials. Chem Eur J 27:1410–1415. https://doi.org/10.1002/chem.202004411
Hu J-X, Jiang X-F, Ma Y-J et al (2021) Optically actuating ultra-stable radicals in a large π-conjugated ligand constructed photochromic complex. Sci China Chem 64:432–438. https://doi.org/10.1007/s11426-020-9891-4
Chai L, Hu Z, Wang X et al (2020) Stringing bimetallic metal–organic framework-derived cobalt phosphide composite for high-efficiency overall water splitting. Adv Sci 7:1903195. https://doi.org/10.1002/advs.201903195
Ma L, Bi Z, Xue Y et al (2020) Bacterial cellulose: an encouraging eco-friendly nano-candidate for energy storage and energy conversion. J Mater Chem A 8:5812–5842. https://doi.org/10.1039/C9TA12536A
Xue H, Chen Q, Jiang F et al (2016) A regenerative metal–organic framework for reversible uptake of Cd(ii): from effective adsorption to in situ detection. Chem Sci 7:5983–5988. https://doi.org/10.1039/C6SC00972G
Qin J-H, Zhang H, Sun P et al (2020) Ionic liquid induced highly dense assembly of porphyrin in MOF nanosheets for photodynamic therapy. Dalton Trans 49:17772–17778. https://doi.org/10.1039/D0DT03031G
Zhang Q, Wei W-J, Li Q et al (2021) Light enhanced proton conductivity in a terbium phosphonate photochromic chain complex. Sci China Chem 64:1170–1176. https://doi.org/10.1007/s11426.021.9976.7
Tan Y, Yang C, Qian W, Teng C (2020) Flower-like MnO2 on layered carbon derived from sisal hemp for asymmetric supercapacitor with enhanced energy density. Journal of Alloys and Compounds 826:154133. https://doi.org/10.1016/j.jallcom.2020.154133
Zhang X, Zhang F, Lu Z et al (2020) Coupling two sequential biocatalysts with close proximity into metal–organic frameworks for enhanced cascade catalysis. ACS Appl Mater Interfaces 12:25565–25571. https://doi.org/10.1021/acsami.0c04317
Harvey PD, Knorr M (2010) Luminescent coordination polymers built upon Cu4X4 (X=Br,I) clusters and mono- and dithioethers. Macromol Rapid Commun 31:808–826. https://doi.org/10.1002/marc.200900893
Shan X, Jiang F, Yuan D et al (2013) A multi-metal-cluster MOF with Cu4I4 and Cu6S6 as functional groups exhibiting dual emission with both thermochromic and near-IR character. Chem Sci 4:1484. https://doi.org/10.1039/c3sc21995j
Beyer MK, Clausen-Schaumann H (2005) Mechanochemistry: the mechanical activation of covalent bonds. Chem Rev 105:2921–2948. https://doi.org/10.1021/cr030697h
Lee Y-A, Eisenberg R (2003) Luminescence tribochromism and bright emission in gold(I) thiouracilate complexes. J Am Chem Soc 125:7778–7779. https://doi.org/10.1021/ja034560k
Ito H, Saito T, Oshima N et al (2008) Reversible mechanochromic luminescence of [(C6F5Au)2 (μ-1,4-diisocyanobenzene)]. J Am Chem Soc 130:10044–10045. https://doi.org/10.1021/ja8019356
Eaton DF (1988) International union of pure and applied chemistry organic chemistry division commission on photochemistry. J Photochem Photobiol, B 2:523–531. https://doi.org/10.1016/10111344(88)850814
Lasanta T, Olmos ME, Laguna A et al (2011) Making the golden connection: reversible mechanochemical and vapochemical switching of luminescence from bimetallic gold–silver clusters associated through aurophilic interactions. J Am Chem Soc 133:16358–16361. https://doi.org/10.1021/ja206845s
Lim SH, Olmstead MM, Balch AL (2011) Molecular accordion: vapoluminescence and molecular flexibility in the orange and green luminescent crystals of the dimer, Au2(μ-bis-(diphenylphosphino)ethane)2Br2. J Am Chem Soc 133:10229–10238. https://doi.org/10.1021/ja2026807
Koshevoy IO, Chang Y-C, Karttunen AJ et al (2012) Modulation of metallophilic bonds: solvent-induced isomerization and luminescence vapochromism of a polymorphic Au–Cu cluster. J Am Chem Soc 134:6564–6567. https://doi.org/10.1021/ja3018994
Hardt HD, Pierre A (1973) Fluorescence thermochromism of pyridine copper iodides and copper iodide. Z Anorg Allg Chem 402:107–112. https://doi.org/10.1002/zaac.19734020113
Perruchas S, Le Goff XF, Maron S et al (2010) Mechanochromic and thermochromic luminescence of a copper iodide cluster. J Am Chem Soc 132:10967–10969. https://doi.org/10.1021/ja103431d
Perruchas S, Tard C, Le Goff XF et al (2011) Thermochromic luminescence of copper iodide clusters: the case of phosphine ligands. Inorg Chem 50:10682–10692. https://doi.org/10.1021/ic201128a
Perruchas S, Desboeufs N, Maron S et al (2012) Siloxanol-functionalized copper iodide cluster as a thermochromic luminescent building block. Inorg Chem 51:794–798. https://doi.org/10.1021/ic200672r
Cauzzi D, Pattacini R, Delferro M et al (2012) Temperature-dependent fluorescence of Cu5 metal clusters: a molecular thermometer. Angew Chem Int Ed 51:9662–9665. https://doi.org/10.1002/anie.201204052
Sun D, Yuan S, Wang H et al (2013) Luminescence thermochromism of two entangled copper-iodide networks with a large temperature-dependent emission shift. Chem Commun 49:6152. https://doi.org/10.1039/c3cc42741b
Wang R-Y, Zhang X, Yu J-H, Xu J-Q (2019) Copper(I)–polymers and their photoluminescence thermochromism properties. Photochem Photobiol Sci 18:477–486. https://doi.org/10.1039/c8pp00474a
Thefioux Y, Cordier M, Massuyeau F et al (2020) Polymorphic copper iodide anions: luminescence thermochromism and mechanochromism of (PPh4)2[Cu2I4]. Inorg Chem 59:5768–5780. https://doi.org/10.1021/acs.Inorgchem.0c00560
Sun D, Zhang L, Lu H et al (2013) Bright-yellow to orange-red thermochromic luminescence of an AgI6–ZnII2 heterometallic aggregate. Dalton Trans 42:3528. https://doi.org/10.1039/c2dt32375c
Effendy DN, C, Fianchini M, et al (2005) The structural definition of adducts of stoichiometry MX:dppx (1:1) M=CuI, AgI, X=simple anion, dppx=Ph2P(CH2)xPPh2, x=3–6. Inorg Chim Acta 358:763–795. https://doi.org/10.1016/j.ica.2004.09.047
Zhang X, Song L, Hong M et al (2014) Luminescent dinuclear copper(I) halide complexes double bridged by diphosphine ligands: Synthesis, structure characterization, properties and TD-DFT calculations. Polyhedron 81:687–694. https://doi.org/10.1016/j.poly.2014.07.034
Petit C, Favre-Reguillon A, Albela B et al (2009) Mechanistic insight into the reduction of tertiary phosphine oxides by Ti(OiPr)4 /TMDS. Organometallics 28:6379–6382. https://doi.org/10.1021/om900747b
Bruker SAINT (2013) v8.34A; Bruker AXS Inc: Madison, WI
Bruker (2007) APEX2, v2014.5−0; Bruker AXS Inc: Madison, WI
Hassan AA, El-Shaieb KMA, El-Aal ASA et al (2016) Synthesis of bis-oxathiaaza[3.3.3] -propellanes via nucleophilic addition of (1,ω-alkanediyl)bis(N’-organylthioureas) on dicyanomethylene-1,3-indanedione. Arkivoc 2016:406–415. https://doi.org/10.24820/ark.5550190.p009.715
Bruker (2014) SADABS, Bruker AXS Inc: Madison, WI
Spek AL (2003) Single-crystal structure validation with the program PLATON. J Appl Crystallogr 36:7–13. https://doi.org/10.1107/S0021889802022112
SHELXTL SG (1997) V5.1, Software Reference Manual; Bruker, AXS, Inc: Madison, WI
Acknowledgements
HM is grateful to PW for participating in the preparation of complex crystals
Funding
We are thankful for financial support from the PAPD of Jiangsu Higher Education Institutions. This work was supported by the National Natural Science Foundation of China (92161121).
Author information
Authors and Affiliations
Contributions
HM and DW wrote the main manuscript text.
Corresponding author
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Miao, H., Wang, P., Huang, Z. et al. Thermochromic photoluminescence of phosphine-supported dinuclear copper‒halide complexes. Struct Chem 34, 2307–2314 (2023). https://doi.org/10.1007/s11224-023-02165-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02165-5