Abstract
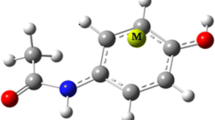
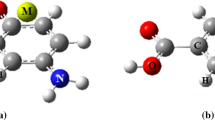
Density functional theory (DFT) calculations were carried out on ML2Cl2 (M = Co, Ni) and M’LCl2 (M’= Zn, Cd) and L=(BDQ) quinolino[3,2-b]benzodiazepine or L = (BDO) = quinolino[3,2-b]benzoxazepine) transition metal complexes. The geometry optimizations and frequency calculations of the aforementioned complexes were performed at the Becke, 3-parameter, Lee–Yang–Parr/triple zeta polarization (B3LYP/TZP) level, followed by the partitioning of the interaction energies using the energy decomposition analysis scheme (EDA) to assess the interaction strength in function of L ligand and the nature of the metal. Indeed, the total bonding energies are more significant for Zn(II) cation in the presence of (QBD) ligand than those of the Cd(II) one and (QBO). The findings show that the electrostatic interaction energy is the most important contributing by two third into the total interaction energy of each of the complex systems. Calculations and analyses were performed by means of the natural bond orbital analyses (NBO) giving rise to significant natural population of the M(II) cations. As concerns of the structures corresponding to odd electron counts, the unpaired electrons are chiefly localized on the metal center with regard to the spin density values. UV–Vis spectra are mainly π→π* electronic transitions corresponding to intra-ligand charge transfers (ILCT) but showed weak influence of the introduction of the MCl2 metallic fragment.









Similar content being viewed by others
Availability of data and materials
The authors make available the data and materials to the readers as free access and without any counterpart.
References
Krishnamoorthy P, Sathyadevi P, Cowley AH, Butorac RR, Dharmaraj N (2011) Evaluation of DNA binding, DNA cleavage, protein binding and in vitro cytotoxic activities of bivalent transition metal hydrazone complexes. Eur J Med Chem 46:3376–3387
Arjmand F, Jamsheera A, Mohapatra DK (2013) Synthesis, characterization and in vitro DNA binding and cleavage studies of Cu(II)/Zn(II) dipeptide complexes. J Photochem Photobiol 121:75–85
Bharti SK, Singh SK (2009) Metal based drugs: current use and future potential. Pharm Lett Title 1:39–51
Manjunath M, Kulkarni AD, Bagihalli GB, Malladi S, Patil SA (2017) Bio-important antipyrine derived Schiff bases and their transition metal complexes: synthesis, spectroscopic characterization, antimicrobial, anthelmintic and DNA cleavage investigation. J Mol Struct 1127:314–21
Hranjec M, Starčević K, Pavelić SK, Lučin P, Pavelić K, Zamola GK (2011) Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. Eur J Med Chem 46:2274–2279
Huang H, Chen Q, Ku X, Meng L, Lin L, Wang X, Zhu C, Wang Y, Chen Z, Li M, Jiang H (2010) A series of d-heterocyclic carboxaldehyde thiosemicarbazones inhibit topoisomerase IId catalytic activity. J Med Chem 53:3048–3064
Badea M, Calu L, Chifiriuc MC, Bleotu C, Marin A, Ion S, Ioniţă G, Stanică N, Măruţescu L, Lazăr V, Marinescu D (2014) Thermal behaviour of some novel antimicrobials based on complexes with a Schiff base bearing 1,2,4-triazole pharmacophore. J Therm Anal Calorim 118:1145–1157
Malik MA, Dar OA, Gull P, Wani MY, Hashmi AA (2018) Heterocyclic Schiff base transition metal complexes in antimicrobial and anticancer chemotherapy. Med Chem Commun 9:409–436
Creaven BS, Duff B, Egan DA, Kavanagh K, Rosair G, Thangella VR, Walsh M (2010) Anticancer and antifungal activity of copper (II) complexes of quinolin-2 (1 H) -one-derived Schiff bases. Inorg Chim Acta 363:4048–4058
Dimiza F, Fountoulaki S, Papadopoulos AN, Kontogiorgis CA, Tangoulis V, Raptopoulou CP, Psycharis V, Terzis A, Kessissoglou DP, Psomas G (2011) Non-steroidal antiinflammatory drug–copper(ii) complexes: structure and biological perspectives. Dalton Trans 40:8555–8568
Uivarosi V (2013) Metal Complexes of Quinolone Antibiotics and Their Applications: An Update. Molecules 18:11153–11197
Imran M, Iqbal J, Iqbal S, Ijaz N (2007) In Vitro antibacterial studies of ciprofloxacinimines and their complexes with Cu (II), Ni (II), Co (II), and Zn (II). Turk J Bio 31:67–72
Silva PP, Guerra W, Silveira JN, Ferreira AM, Bortolotto T, Fischer FL, Terenzi H, Neves A, Pereira-Maia EC (2011) Two new ternary complexes of copper (II) with tetracycline or doxycycline and 1, 10-phenanthroline and their potential as antitumoral: cytotoxicity and DNA cleavage. Inorg Chem 50:6414–6424
Basavaraju B, Bhojya Naik HS, Prabhakara MC (2007) Transition metal complexes of quinolino[3,2-b] benzodiazepine and quinolino[3,2-b]benzoxazepine: synthesis, characterization, and antimicrobial studies. Bioinorg Chem 1–6
Bringmann G, Reichert Y, Kane VV (2004) The total synthesis of streptonigrin and related antitumor antibiotic natural products. Tetrahedron 16:3539–3574
Gandra RM, Mc Carron P, Fernandes MF, Ramos LS, Mello TP, Aor AC, Branquinha MH, McCann M, Devereux M, Santos AL (2017) Antifungal potential of copper (II), manganese (II) and silver (I) 1, 10-phenanthroline chelates against multidrug-resistant fungal species forming the Candida haemulonii complex: impact on the planktonic and biofilm lifestyles. Front Microbiol 8:1257
Farah S, Bouchakri N, Zendaoui SM, Saillard JY, Zouchoune B (2010) Electronic structure of bis-azepine transition-metal complexes: a DFT investigation. J Mol Struc 953:143–150
Farah S, Ababsa S, Benhamada N, Zouchoune B (2010) Theoretical investigation of the coordination of dibenzazepine to transition- metal complexes: a DFT study. Polyhedron 29:2722–2730
Benmachiche A, Zendaoui SM, Bouaoud SE, Zouchoune B (2012) Electronic structure and coordination chemistry of phenanthridine ligand in frst-row transition metal complexes: a DFT study. Int J Quant Chem 113:985–999
Chekkal F, Zendaoui SM, Zouchoune B, Saillard JY (2013) Structural and spin diversity of M (indenyl) 2 transition-metal complexes: a DFT investigation. New J Chem 37:2293–2302
Farah S, Korichi H, Zendaoui SM, Saillard JY, Zouchoune B (2009) The coordination of azepine to transition-metal complexes: a DFT analysis. Inorg Chim Acta 362:3541–3546
Zendaoui SM, Zouchoune B (2016) Coordination chemistry of mixed M(benzene)(cyclopendadienyl) sandwich complexes: electronic properties and bonding analysis. New J Chem 40:2554–2564
Saiad A, Zouchoune B (2015) Electronic structure and bonding analysis of transition metal sandwich and half-sandwich complexes of triphenylene ligand. Can J Chem 93:1096–1108
Bouchakri N, Benmachiche A, Zouchoune B (2011) Bonding analysis and electronic structure of the transition-metal benzoquinoline complexes: theoretical study. Polyhedron 30:2644–2653
Wang H, Xie Y, Dumitrescu IS, King RB, Schaefer HF III (2009) The mixed sandwich compounds C5H5MC7H7 of the frst row transition metals: variable hapticity of the seven-membered ring. Mol Phys 108:883–894
Fan Q, Feng H, Sun W, Li H, Xie Y, King RB, Scharfer HF III (2013) A new type of sandwich compound: homoleptic bis(trimethylenemethane) complexes of the first row transition metals. New J Chem 37:1545–1953
Zouchoune B, Mansouri L (2017) Electronic structure and UV– Vis spectra simulation of square planar bis(1-(4-methylphenylazo)- 2-naphtol)-transition metal complexes [M(L)2]x (M = Ni, Pd, Pt, Cu, Ag). Struct Chem 30:691–701
Mansouri L, Zouchoune B (2015) Electronic properties and substitution effects of the (2-phenylazo-l-naphthyl) Azo dye species: a TD-DFT electronic spectra investigation. Can J Chem 93:509–517
Drideh S, Zouchoune B, Zendaoui SM, Saillard JY (2018) Electronic structure and structural diversity in indenyl in heterobinuclear transition-metal half-sandwich complexes S. Theor Chem Acc 137:1–12
Chetioui S, Zouchoune B, Merazig H, Bouaoud SE, Rouag D, Djukic JP (2021) Synthesis, spectroscopic characterization, crystal structure and theoretical investigation of two azo-palladium (II) complexes derived from substituted (1-phenylazo)-2-naphtol. Transit Met Chem 46:91–101
Nemdili H, Zouchoune B, Zendaoui MS, Ferhati A (2019) Structural, bonding and redox properties of 34-electron bimetallic complexes and their oxidized of 32- and 33-electrons and their reduced of 35- and 36-electrons derivatives containing the indenyl fused π-system: a DFT overview. Polyhedron 160:219–228
Podunavac-Kuzmanović SO, Vojinović LS (2003) Synthesis and physico-chemical characterization of zinc (II), nickel (II) and cobalt (II) complexes with 2-phenyl-2-imidazoline. Acta Period Technol 34:119–124
Araujo CM, Doherty MD, Konezny SJ, Luca OR, Usyatinsky A, Grade H, Lobkovsky E, Soloveichik GL, Crabtree RH, Batista VS (2012) Tuning redox potentials of bis(imino)pyridine cobalt complexes: an experimental and theoretical study involving solvent and ligand effects. Dalton Trans 41:3562–3573
Jiang F, Siegler MA, Sun X, Jiang L, Fonseca Guerra C, Bouwman E (2018) Redox interconversion between cobalt(III) thiolate and cobalt(II) disulfide compounds. Inorg Chem 57:8796–8805
Ramadthan EM (2013) Synthesis and characterization of new some first transition metals complexes of schiff base N, N’-bis[2-methylenethiophene]-1,2- phenylenediimine. Irq Nat J Chem 50:154–166
Gruff ES, Koch SA (1989) A Trigonal Planar [Zn(SR)3]1- Complex. A possible new coordination mode for zinc-cysteine centers. J Am Chem Soc 111:8762–8763
Power PP, Shoner SC (1990) Synthesis and structure of [Et2OZn (SC6H2tBu3) 2], the first T-shaped zinc complex. Angew Chem Int Ed Engl 29:1403–1404
Darensbourg DJ, Niezgoda SA, Draper JD, Reibenspies JH (1999) Trigonal-planar zinc (II) and cadmium (II) tris (phenoxide) complexes. Inorg Chem 38:1356–1359
Conradie J, Ghosh A (2007) Electronic structure of trigonal-planar transition-metal− imido complexes: spin-state energetics, spin-density profiles, and the remarkable performance of the OLYP functional. J Chem Theory Comput 3:689–702
Kogut E, Wiencko HL, Zhang L, Cordeau DE, Warren TH (2005) A terminal Ni (III)− imide with diverse reactivity pathways. J Am Chem Soc 127:11248–11249
Park S, Lee JK, Lee H, Nayab S, Shin JW (2019) Zinc (II), palladium (II) and cadmium (II) complexes containing 4-methoxy-N-(pyridin-2-ylmethylene) aniline derivatives: Synthesis, characterization and methyl methacrylate polymerization. Appl Organometal Chem 33:e4797
Morokuma K (1971) Molecular orbital studies of hydrogen bonds. III. C= O··· H-O hydrogen bond in H2CO··· H2O and H2CO··· 2H2O. J Chem Phys 55:1236–1244
Ziegler T, Rauk A (1979) Carbon monoxide, carbon monosulfde, molecular nitrogen, phosphorus trifuoride, and methyl isocyanide as. sigma. donors and. pi. acceptors. A theoretical study by the Hartree-Fock-Slater transition-state method. Inorg Chem 18:1755–1759
Ziegler T, Rauk A (1979) A theoretical study of the ethylenemetal bond in complexes between copper (1+), silver (1+), gold (1+), platinum (0) or platinum (2+) and ethylene, based on the Hartree-Fock-Slater transition-state method. Inorg Chem 18:1558–1565
Zaiter A, Zouchoune B (2018) Electronic structure and energy decomposition of binuclear transition metal complexes containing β-diketiminate and imido ligands: spin state and metal’s nature effects 29:1307–1320
Tabrizi L, Zouchoune B, Zaiter A (2018) Experimental and theoretical investigation of cyclometallated platinum(II) complex containing adamantanemethylcyanamide and 1,4-naphthoquinone derivative as ligands). RSC Adv 9:287–300
Tabrizi L, Zouchoune B, Zaiter A (2019) Theoretical and experimental study of gold(III), palladium(II), and platinum (II) complexes with 3-((4-nitrophenyl)thio)phenylcyanamide and 2,2′-bipyridine ligands: cytotoxic activity and interaction with 9-methylguanine. Inorg Chim Act 499:119211
Khireche M, Zouchoune B, Ferhati A, Nemdili H, Zerizer MA (2021) Understanding the chemical bonding in sandwich complexes of transition metals coordinated to nine-membered rings: energy decomposition analysis and the donor–acceptor charge transfers. Theor Chem Acc 140:1–17
Frenking G, Froehlich N (2000) The nature of the bonding in transition-metal compounds. Chem Rev 100:717–774
Zerizer MA, Nemdili H, Zouchoune B (2022) Electron transfers’ assessment between stannol ring of triple-decker complexes and M (CO) 5 (M= Cr, Mo, W), MnCp (CO) 2 and CoCp (CO) metallic fragments: Bonding and energy decomposition analysis. Polyhedron 223:115960
Mecheri S, Zouchoune B, Zendaoui SM (2020) Bonding and electronic structures in dinuclear (X)[(Ind) M2L2] complexes (M= Ni, Pd, L= CO, PEt3, X= Cl, Allyl, Ind= indenyl, Cp= cyclopentadienyl): analogy between four-electron donor ligands. Theor Chem Acc 139:1–13
Mecheri S, Zouchoune B (2023) Terminal and bridging ligand effects on M(I)-M(I) multiple bonding: a DFT investigation of the coordination in (X)[M2Cl]L2 complexes (M = Cr, Fe, L = CO, PEt3, X = Cl, allyl, Cp and indenyl). Inter J Quant Chem e27089
Frenking G, Wichmann K, Fröhlich N, Grobe J, Golla W, Van DL, Krebs B, Läge M (2002) Nature of the metal− ligand bond in M (CO) 5PX3 complexes (M= Cr, Mo, W; X= H, Me, F, Cl): synthesis, molecular structure, and quantum-chemical calculations. Organometallics 21:2921
Mokrane Z, Zouchoune B, Zaiter A (2020) Coordination’s preference and electronic structure of N-heterocyclic carbene–monometallic complexes: DFT evaluation of σ-bonding and π-backbonding interactions. Theor Chem Acc 139:1–14
Jacquemin D, Perpète EA, Ciofini I, Adamo C (2008) On the TD-DFT UV/vis spectra accuracy: the azoalkanes. Theo Chim Acc 120:405–410
Jacquemin D, Preat J, Perpète EA, Vercauteren DP, André JM, Ciofini I, Adamo C (2011) Absorption spectra of azobenzenes simulated with time-dependent density functional theory. Int J Quant Chem 111:4224–4240
Höfener S, Trumm M, Koke C, Heuser J, Ekström U, Skerencak-Frech A, Schimmelpfennig B, Panak PJ (2016) Computing UV/vis spectra using a combined molecular dynamics and quantum chemistry approach: bis-triazin-pyridine (BTP) ligands studied in solution. Phys Chem Chem Phys 18:7728–7736
Lu JS, Ko SB, Walters NR, Kang Y, Sauriol F, Wang S (2013) Formation of azaborines by photoelimination of B, N-heterocyclic compounds. Angew Chem 125:4642–4646
ADF2021, SCM, Theoretical chemistry. Vrije Universiteit, Amsterdam. www.scm.com
Baerends EJ, Ellis DE, Ros P (1973) Self-consistent molecular Hartree–Fock–Slater calculations The computational procedure. Chem Phys 2:41–51
te Velde G, Baerends EJ (1992) Numerical integration for polyatomic systems. J Comput Phys 99:84–98
Fonseca Guerra C, Snijders JG, te Velde G, Baerends EJ (1998) Towards an order-N DFT method. Theor Chem Acc 99:391–403
Bickelhaupt FM, Baerends EJ (2000) Kohn-Sham DFT: predicting and understanding chemistry. Rev Comput Chem 15:1–86
te Velde G, Bickelhaupt FM, Fonseca Guerra C, van Gisbergen SJA, Baerends EJ, Snijders JG, Ziegler T (2001) Chemistry with ADF. J Comput Chem 22:931–967
Becke AD (1993) Density-functional thermochemistry III. The role of exact exchange. J Chem Phys 98:5642–5648
Lee C, Yang W, Parr RG (1998) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Versluis L, Ziegler T (1988) The determination of molecular structures by density functional theory. The evaluation of analytical energy gradients by numerical integration. J Chem Phys 88:322–329
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on main group molecules. Chem Phys 96:9005–9012
Fan L, Ziegler T (1992) Application of density functional theory to infrared absorption intensity calculations on transition-metal carbonyls. Phys Chem 96:6937–6941
Runge E, Gross EK (1984) Density-functional theory for time-dependent systems. Phys Rev Lett 52:997
Klamt A, Schüümann G (1993) COSMO: a new approach to dielectric screening in solvents with explicit expressions for the screening energy and its gradient. J Chem Soc Perkin Trans 2:799–805
Funding
The authors are grateful to the Algerian MESRS (Ministère de l’Enseignement Supérieur et de la Recherche Scientifique) and the Algerian DGRSDT (Direction Générale de la Recherche Scientifique et du Développement Technologique) for the financial support.
Author information
Authors and Affiliations
Contributions
Yasmina Hafsi: execution of DFT calculations, results collection, and conceptualization. Sabri Mecheri: writing original draft. Bachir Zouchoune: conceptualization, manuscript writing, and validation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hafsi, Y., Mecheri, S. & Zouchoune, B. Molecular and electronic structures, bonding analysis, and UV–Vis spectra predictions of quinolino[3,2-b]benzodiazepine and quinolino[3,2-b]benzoxazepine metal transition M(L)2Cl2 and M(L)Cl2 complexes. Struct Chem 34, 2051–2064 (2023). https://doi.org/10.1007/s11224-023-02145-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-023-02145-9