Abstract
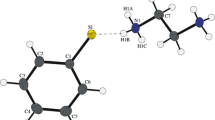
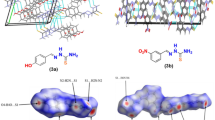
Crystal and electronic structures of a newly synthesized thiazolium pentaiodide, C3H4NS(I5), were examined in detail. The title pentaiodide crystallizes in the monoclinic space group P21/m with the unit cell volume of 1289.27(6) Å3. Its crystal structure features branched pentaiodide chains composed of alternating I3− and I2 building units, whereas the chains are further linked into a 3D array by thiazolium cations with the help of (N)H⋅⋅⋅I and S⋅⋅⋅I bonds. The electronic structure and bonding assessed by DFT calculations show that covalent interactions within the I2 and I3− units are supplemented by non-covalent (N)H⋅⋅⋅I and S⋅⋅⋅I interactions, which were revealed by the electron localization function and reduced density gradient analyses.






Similar content being viewed by others
Availability of data and materials
Data are available from the authors upon a reasonable request.
Code availability
Details on the crystal structure investigation may be obtained from the Cambridge Crystallographic Data Centre by quoting the CCDC number 2191074.
References
Savastano M (2021) Words in supramolecular chemistry: the ineffable advances of polyiodide chemistry. Dalton Trans 50:1142–1165
Udalova NN, Tutantsev AS, Fateev SA, Zharenova EA, Belich NA, Nemygina EM, Ryabova AV, Goodilin EA, Tarasov AB (2021) Crystallization features of MAPbI3 hybrid perovskite during the reaction of PbI2 with reactive polyiodide melts. Russ J Inorg Chem 66:153–162
Belich NA, Petrov AA, Rudnev PO, Stepanov NM, Turkevych I, Goodilin EA, Tarasov AB (2020) From metallic lead films to perovskite solar cells through lead conversion with polyhalides solutions. ACS Appl Mater Interfaces 12:20456–20461
Starkholm A, Kloo L, Svensson PH (2019) Polyiodide hybrid perovskites: a strategy to convert intrinsic 2D systems into 3D photovoltaic materials. ACS Appl Energy Mater 2:477–485
Voronin OS, Grishko AY, Finkelberg YM, Petrov AA, Goodilin EA, Tarasov AB (2022) Iodine solution treatment in nonpolar solvent as a facile approach to improve morphology and photostability of perovskite films. J Phys Chem Let 13:2695–2703
Shestimerova TA, Bykov AV, Kuznetsov AN, Grishko AY, Wei Z, Dikarev EV, Shevelkov AV (2022) Pattern of covalent and non-covalent interactions within pentaiodide anion in the structure of (3-HOC5H9NH2)I5. Z Anorg Allg Chem 648:e202200039
Yushina ID, Batalov VI, Bartashevich EV, Davydov AO, Zelenovskiy PS, Masunov AJ (2017) Raman spectroscopy and theoretic study of hyperpolarizability effect in diiodobutenyl-bis-thioquinolinium triiodide at low temperature. Raman Spectrosc 48:1411–1413
Svensson PH, Kloo L (2003) Synthesis, structure, and bonding in polyiodide and metal iodide−iodine systems. Chem Rev 103:1649–1684
Shestimerova TA, Shevelkov AV (2018) Metal-inorganic frameworks with pnictogen linkers. Russ Chem Rev 87(1):28–48
Kloo L, Rosdahl J, Svensson PH (2002) On the intra-and intermolecular bonding in polyiodides. Eur J Inorg Chem 5:1203–1209
Yelovik NA, Mironov AV, Bykov MA, Kuznetsov AN, Grigorieva AV, Wei Z, Dikarev EV, Shevelkov AV (2016) Iodobismuthates containing one-dimensional BiI4– anions as prospective light-harvesting materials: synthesis, crystal and electronic structure, and optical properties. Inorg Chem 55:4132–4140
Andreev IA, Ratmanova NK, Augustin AU, Ivanova OA, Levina II, Khrustalev VN, Werz DB, Trushkov IV (2021) Protic ionic liquid as reagent, catalyst, and solvent: 1-methylimidazolium thiocyanate. Angew Chem Int Ed 60:7927–7934
Andreev IA, Boichenko MA, Ratmanova NK, Ivanova OA, Levina II, Khrustalev VN, Sedov IA, Trushkov IV (2022) 4-(Dimethylamino)pyridinium azide in protic ionic liquid media as a stable equivalent of hydrazoic acid. Adv Synth Catal 364:2403–2415
Bruker Corporation (1997) SMART (control) and SAINT (integration) software; Version 5.0; Bruker AXS Inc.: Madison, WI, USA
Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D (2015) Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J Appl Cryst 48:3–10
Dolomanov OV, Bourhis LJ, Gildea RJ, Howard JAK, Puschmann H (2009) OLEX2: a complete structure solution, refinement and analysis program. J Appl Cryst 42:339–341
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Cryst Sect C Struct Chem 71:3–8
Kresse G, Joubert D (1999) From ultrasoft pseudopotentials to the projector augmented-wave method. Phys Rev B 59:1758–1775
Kresse G, Furthmüller J, Vienna J. Ab initio simulation package (VASP), v.5.4.4, http://www.vasp.at/
Perdew JP, Burke K, Ernzerhof M (1996) Generalized gradient approximation made simple. Phys Rev Lett 77:3865
Grimme S, Antony J, Ehrlich S, Krieg S (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104
Monkhorst HJ, Pack JD (1976) Special points for Brillouin-zone integrations. Phys Rev B 13:5188–5192
Grimme S, Hansen A, Ehlert S, Mewes JM (2021) r2SCAN-3c: a “Swiss army knife” composite electronic-structure method. J Chem Phys 154:064103
Metz B, Stoll H, Dolg M (2000) Small-core multiconfiguration-Dirac–Hartree–Fock-adjusted pseudopotentials for post-d main group elements: application to PbH and PbO. J Chem Phys 113:2563–2569
Rappoport D, Furche F (2010) Property-optimized Gaussian basis sets for molecular response calculations. J Chem Phys 133:134105
Neese F, Wiley F (2012) The ORCA program system. Interdisciplinary Reviews: Computational Molecular Science 2:73–78
Neese F, Wennmohs F, Becker U, Riplinger C (2020) The ORCA quantum chemistry program package. J Chem Phys 152:224108
Becke AD, Edgecombe KE (1990) A simple measure of electron localization in atomic and molecular systems. J Chem Phys 92:5397–5403
Kohout M, Savin A (1996) Atomic shell structure and electron numbers. Int J Quantum Chem 60:875–882
Savin A, Jepsen O, Flad J, Andersen OK, Preuss H, Schnering HG (1992) Electron localization in solid-state structures of the elements: the diamond structure. Angew Chem Int Ed Engl 31:187–188
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Molec Graphics 14(1):33–38
Ganose AM, Jackson AJ, Scanlon DO (2018) sumo: command-line tools for plotting and analysis of periodic* ab initio* calculations. J Open Source Softw 3:717
Tebbe KF, Bittner M (1995) Untersuchungen an Polyhalogeniden. XVI. Darstellung und Kristallstrukturen der Bipyridiniumpolyiodide Bipy· HIn mit n= 3, 5 und 7. Z Anorg Allg Chem 621:218–224
Usoltsev NA, Korobeinikov NA, Sokolov MN, Adonin SA (2021) Synthesis and crystal structures of polyhalide salts of copper(I) complexes [Cu(CH3CN)4]Br 5 and [Cu(CH3CN)4]I5. Russ J Inorg Chem 66:1477–1481
Yushina ID, Tarasova NM, Kim DG, Sharutin VV, Bartashevich EV (2019) Noncovalent bonds, spectral and thermal properties of substituted thiazolo [2, 3-b][1, 3] thiazinium triiodides. Crystals 9:506
Evans HA, Andrews JL, Fabini DH, Preefer MB, Wu G, Cheetham AK, Wudl F, Seshadri R (2019) The capricious nature of iodine catenation in I2 excess, perovskite-derived hybrid Pt(IV) compounds. Chem Commun 55:588–591
Shestimerova TA, Bykov MA, Wei Z, Dikarev EV, Shevelkov AV (2019) Crystal structure and two-level supramolecular organization of glycinium triiodide. Russ Chem Bull Int Ed 68:520–1524
Poreba T, Ernst M, Zimmer D, Macchi P, Casati N (2019) Pressure-induced polymerization and electrical conductivity of a polyiodide. Angew Chem Int Ed 58:6625–6629
Bolhuis F, Koster PB, Migchelsen T (1967) Refinement of the crystal structure of iodine at 110 K. Acta Crystallogr 23:90–91
Shestimerova TA, Mironov AV, Bykov MA, Grigorieva AV, Wei Z, Dikarev EV, Shevelkov AV (2020) Assembling polyiodides and iodobismuthates using a template effect of a cyclic diammonium cation and formation of a low-gap hybrid iodobismuthate with high thermal stability. Molecules 25:2765
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506
Funding
This work was supported by the Russian Foundation for Basic Research, grant no. 21–53-50007. The synthesis and characterization of starting thiazolium thiocyanate were performed during realization of project 21–73-10212 supported by the Russian Science Foundation.
Author information
Authors and Affiliations
Contributions
TAS performed the crystal structure solution and co-wrote the manuscript; IAA, NKR, and IVT performed synthesis and primary characterization and co-wrote the manuscript; ANK performed calculations and co-wrote the manuscript; and AVS supervised the research and co-wrote and edited the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shestimerova, T.A., Andreev, I.A., Ratmanova, N.K. et al. Crystal and electronic structure of thiazolium pentaiodide: an experimental and theoretical study of covalent and non-covalent bonds. Struct Chem 34, 1557–1564 (2023). https://doi.org/10.1007/s11224-022-02097-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02097-6