Abstract
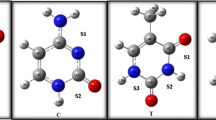
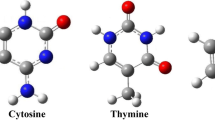
The biologically complex configurations paired of methimazole (MET) anti-thyroid drug with each of the three nucleic acid bases of single ring based on pyrimidine (uracil (U), thymine (T) and cytosine (C)) have been studied at different levels of density functional theory. Twelve double H-bonded complexes were proposed and designed stand on the data obtained from electrostatic potential (ESP) maps of the studied nucleic acid bases and MET drug. The interaction energies of the optimized structures of all three set of complexes (MET-U, MET-T and MET-C) were computed using M062X/AUG–cc–pVDZ computational level and then corrected using M062X–GD3 functional with the same basis sets. The corrected interaction energies for the of MET-U, MET-T and MET-C complex series range from −10.89 to −17.52, −11.31 to −17.62, and −12.33 to −19.54 kcal mol−1, respectively. The highest interaction energy has been calculated between C nucleobase and the MET drug. Structural geometries of the U, T and C nucleobases as well as MET drug show that they can act as H-bond donor and H-bond acceptor simultaneously. The existence, strength and nature of the hydrogen bond interactions in the complex were confirmed and investigated using vibrational frequency, natural bond orbital (NBO) and non-covalent interactions (NCI) analysis and also with quantum theory of atoms in molecules (QTAIM) analysis at M062X/AUG–cc–pVDZ level of theory. In the most stable complexes of each series, charge transfer occurs (CT) from MET drug to the U, T and C nucleobases. Analysis of the electron density property shows that the N1− H7···Sʹ6 hydrogen bond nature in the most stable MET-UR1R′1, MET-TR1R′1 and MET-CR1R′1 complexes is slightly covalent and the nature of other hydrogen bonds in three series of studied complexes is electrostatic.




















Similar content being viewed by others
Data Availability
The online version of this article will be available at … (After acceptance).
References
Watson JD, Crick FHC (1993) Molecular structure of nucleic acids. JAMA 269:1966–1967. https://doi.org/10.1001/jama.1993.03500150078030
Krause H, Ernstberger B, Neusser HJ (1991) Binding energies of small benzene clusters. Chem Phys Lett 184:411–417. https://doi.org/10.1016/0009-2614(91)80010-U
Neusser HJ, Krause H (1994) Binding energy and structure of van der Waals complexes of benzene. Chem Rev 94:1829–1843. https://doi.org/10.1021/cr00031a005
Rutledge LR, Campbell-Verduyn LS, Hunter KC, Wetmore SD (2006) Characterization of nucleobase–amino acid stacking interactions utilized by a DNA repair enzyme. J Phys Chem B 110:19652–19663. https://doi.org/10.1021/jp061939v
Rutledge LR, Campbell-Verduyn LS, Wetmore SD (2007) Characterization of the stacking interactions between DNA or RNA nucleobases and the aromatic amino acids. Chem Phys Lett 444:167–175. https://doi.org/10.1016/j.cplett.2007.06.090
Gonza´lez Moa MJ, Mandado M, Mosquera RA, (2007) A computational study on the stacking interaction in quinhydrone. J Phys Chem A 111:1998–2001. https://doi.org/10.1021/jp0661701
Mignon P, Loverix S, De Proft F, Geerlings P (2004) Influence of stacking on hydrogen bonding: quantum chemical study on pyridine−benzene model complexes. J Phys Chem A 108:6038–6044. https://doi.org/10.1021/jp049240h
Quinonero D, Frontera A, Deya PM, Alkorta I, Elguero J (2008) Interaction of positively and negatively charged aromatic hydrocarbons with benzene and triphenylene: towards a model of pure organic insulators. Chem Phys Lett 460:406–410. https://doi.org/10.1016/j.cplett.2008.06.028
Asakawa M, Ashton PR, Hayes W, Janssen HM, Meijer EW, Menzer S, Pasini D, Stoddart JF, White AJP, Williams DJ (1998) Constitutionally asymmetric and chiral [2] pseudorotaxanes. J Am Chem Soc 120:920–931. https://doi.org/10.1021/ja970018i
Sponer J, Leszczynski J, Hobza P (2002) Electronic properties, hydrogen bonding, stacking, and cation binding of DNA and RNA bases. J Biopolymers 61:3–31. https://doi.org/10.1002/1097-0282(2001)61:1%3c3::AID-BIP10048%3e3.0.CO;2-4
Hunter AC, Sanders JKM (1990) The nature of.pi.–.pi. interactions. J Am Chem Soc 112:5525–5534. https://doi.org/10.1021/ja00170a016
Sponer J, Jurecka P, Hobza P (2004) Accurate interaction energies of hydrogen-bonded nucleic acid base pairs. J Am Chem Soc 126:10142–10151. https://doi.org/10.1021/ja048436s
Gu J, Wang J, Leszczynski J, Xie Y, Schaefer HF (2008) To stack or not to stack: performance of a new density functional for the uracil and thymine dimers. Chem Phys Lett 459:164–166. https://doi.org/10.1016/j.cplett.2008.05.049
Cysewski P, Czyznikowska Z, Zalesny R, Czelen P (2008) The post-SCF quantum chemistry characteristics of the guanine-guanine stacking B-DNA. Phys Chem Phys 10:2665–2672. https://doi.org/10.1039/B718635E
Esrafili MD, Behzadi H, Beheshtian J, Hadipour NL (2008) Theoretical 14N nuclear quadrupole resonance parameters for sulfa drugs: sulfamerazine and sulfathiazole. J Mol Graph Model 27:326–331. https://doi.org/10.1016/j.jmgm.2008.05.007
Solimannejad M, Rezaei Z, Esrafili MD (2013) Competition and interplay between the lithium bonding and hydrogen bonding: R3C···HY···LiY and R3C···LiY···HY triads as a working model (R=H, CH3; Y=CN, NC). J Mol Model 19:5031–5035. https://doi.org/10.1007/s00894-013-2006-6
Vojta D, Vazdar M (2014) The study of hydrogen bonding and π–π interactions in phenol⋯ethynylbenzene complex by IR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 132:6–14. https://doi.org/10.1016/j.saa.2014.04.149
Esrafili MD, Amiri Z, Shankal F (2016) Strong cooperative effects between π-hole and dihydrogen bonds interactions: a computational study. Mol Phys 114:2315–2324. https://doi.org/10.1080/00268976.2016.1203037
Afzali R, Vakili M, Nekoei AR, Tayyari SF (2014) Intramolecular hydrogen bonding and vibrational assignment of 1,1,1-trifluoro-5, 5-dimethyl-2,4-hexanedione. J Mol Struct 1076:262–271. https://doi.org/10.1016/j.molstruc.2014.07.059
Esrafili MD, Behzadi H (2012) A theoretical study on H-bonding interactions in maleic acid: calculated 17O, 1HNMR parameters and QTAIM analysis. Mol Simul 38:896–905. https://doi.org/10.1080/08927022.2012.669477
Vojta D, Matanović I, Kovačević G, Baranović G (2014) The study of secondary effects in vibrational and hydrogen bonding properties of 2- and 3-ethynylpyridine and ethynylbenzene by IR spectroscopy. Spectrochim Acta A Mol Biomol Spectrosc 132:215–224. https://doi.org/10.1016/j.saa.2014.04.166
Esrafili MD, Vakili M (2017) The effect of hydrogen-bonding cooperativity on the strength and properties of σ-hole interactions: an ab initio study. Mol Phys 115:913–924. https://doi.org/10.1080/00268976.2017.1292013
Rozas I, Alkorta I, Elguero J (2004) Modelling protein-RNA interactions: an electron density study of the formamide and formic acid complexes with RNA bases. J Phys Chem B 108:3335–3341. https://doi.org/10.1021/jp036901m
Kasende OE, Nziko VPN, Scheiner S (2016) Interactions of nucleic acid bases with temozolomide. Stacked, perpendicular, and coplanar heterodimers. J Phys Chem B 120:9347–9361. https://doi.org/10.1021/acs.jpcb.6b06150
Sponer J, Leszczynski J, Hobza P (2002) Electronic properties, hydrogen bonding, stacking, and cation binding of DNA and RNA bases. Biopolymers 61:3–31. https://doi.org/10.1002/1097-0282(2001)61:1%3c3::AID-BIP10048%3e3.0.CO;2-4
Garrett RH, Grisham CM (2001) Principals of biochemistry with a human focus. Brooks/Cole, United States
Klein D, Moore P, Steitz T (2004) The roles of ribosomal proteins in the structure assembly, and evolution of the large ribosomal subunit. J Mol Biol 340:141–177. https://doi.org/10.1016/j.jmb.2004.03.076
Serganov A, Nudler E (2013) A decade of riboswitches. Cell 152:17–24. https://doi.org/10.1016/j.cell.2012.12.024
Yang Z, Rodgers MT (2004) Influence of halogenation on the properties of uracil and its noncovalent interactions with alkali metal ions. Threshold collision-induced dissociation and theoretical studies. J Am Chem Soc 126:16217–16226. https://doi.org/10.1021/ja045375p
Brown DJ (1994) The chemistry of heterocyclic compounds, vol 52. Wiley-Interscience
Podolyan Y, Gorb L, Leszczynski J (2003) Ab initio study of the prototropic tautomerism of cytosine and guanine and their contribution to spontaneous point mutations. Int J Mol Sci 4:410–421. https://doi.org/10.3390/i4070410
Muniz-Miranda M, Muniz-Miranda F, Pedone A (2016) Raman and DFT study of methimazole chemisorbed on gold colloidal nanoparticles. Phys Chem Chem Phys 18:5974–5980. https://doi.org/10.1039/C5CP07597A
Weetman AP, McGregor AM, Hall R (1984) Evidence for an effect of antithyroid drugs on the natural history of Graves’ disease. Clin Endocrinol 21:163–172. https://doi.org/10.1111/j.1365-2265.1984.tb03456.x
Kendall-Taylor P (1984) Are antithyroid drugs immunosuppressive? Br Med J 288:509–511. https://doi.org/10.1136/bmj.288.6416.509
Laurence C, Ghomari MJEl, Berthelot M, (1998) Structure and molecular interactions of anti-thyroid drugs. Part 2. Electron donor properties of carbimazole. J Chem Soc Perkin Trans 2:1163–1166. https://doi.org/10.1039/A800902C
Laurence C, Ghomari MJEl, Le Questel JY, Berthelo M, Mokhlisse R, (1998) Structure and molecular interactions of anti-thyroid drugs. Part 3. 1 Methimazole: a diiodine sponge. J Chem Soc Perkin Trans 2:1545–1552. https://doi.org/10.1039/A803002B
Pradhan S, Sarma H, Bharadwaz B, Satish Kumar Mattaparthi V (2017) Comparative study on the binding affinity of methimazole and propylthiouracil to thyroid peroxidase as an anti-thyroid drug: an in silico approach. Mol Imag Dynamic 7:1–9. https://doi.org/10.4172/2155-9937.1000131
Shakourian-Fard M, Ghenaatian HR, Alizadeh V, Kamath G, Khalili B (2021) Density functional theory investigation into the interaction of deep eutectic solvents with amino acids. J Mol Liq 343:117624. https://doi.org/10.1016/j.molliq.2021.117624
Khalili B, Rimaz M (2018) A quantum chemical study of the interactions of uracil as a constituent of ribonucleic acid (RNA) with thiazolidinedione and rhodanine bioactive molecules: an insight into energetic and structural features. Struct Chem 29:681–702. https://doi.org/10.1007/s11224-017-1062-4
Mohammadi A, Khalili B, Saberi Haghayegh A (2019) A novel chromone based colorimetric sensor for highly selective detection of copper ions: synthesis, optical properties and DFT calculations. Spectrochim Acta Part A Mol Biomol Spectrosc 222:117193–117201. https://doi.org/10.1016/j.saa.2019.117193
Khalili B, Rimaz M, Tondro T (2015) DFT study on Foscarnet as an antiviral drug: conformer analysis, gas phase acidity, metal ion affinity and influence of metal complexation on gas phase acidity. J Mol Struct 1080:80–87. https://doi.org/10.1016/j.molstruc.2014.09.075
Khoshsoroor S, Mohammadi A, Khalili B, Mohammadi S (2020) A novel uracil-based chemosensor for sequential detection of copper (II) and cyanide ions and its application in real samples. J Photochem Photobiol, A 388:112208–112243. https://doi.org/10.1016/j.jphotochem.2019.112208
Talaei R, Khalili B, Mokhtary M (2020) Modulation of opto-electronic properties of the functionalized hexagonal boron nitride nanosheets with tunable aryl alkyl ionic liquids (TAAILs): Defect based analysis. J Mol Liq 304:112696–112711. https://doi.org/10.1016/j.molliq.2020.112696
Khalili B, Rimaz M (2017) An investigation on the physicochemical properties of the nanostructured [(4-X)PMAT] [N(CN)2] ion pairs as energetic and tunable aryl alkyl amino tetrazolium based ionic liquids. J Mol Struct 1137:530–542. https://doi.org/10.1016/j.molstruc.2017.02.053
Khalili B, Rasoulian M, Ghauri K (2020) First time investigation of the substitution effect at anion part of the ILs on their physicochemical properties using [DMT][4-XPhSO3] (X=NH2, OH, H, F, Br, CHO, CF3, CN and NO2) as a model ILs: a systematic DFT study. J Mol Struct 1201:127171–127191. https://doi.org/10.1016/j.molstruc.2019.127171
Khalili B, Rimaz M (2017) Does interaction between an amino acid anion and methylimidazolium cation lead to a nanostructured ion pairs of [Mim][AA] as an ionic liquid? J Mol Liq 229:267–277. https://doi.org/10.1016/j.molliq.2016.12.077
Khalili B, Mamaghani M, Bazdid-Vahdati N (2022) Structural design and physicochemical specifications exploring of the new di-cationic ionic liquids (D-ILs) composed of para-xylyl linked N-methylimidazolium cation and various anions: a full M06–2X computational study. Theoret Chem Acc 141:1–18. https://doi.org/10.1007/s00214-021-02862-6
Khalili B, Moradpour M (2022) Fluorination effects on the physicochemical properties of the nanostructured tunable ionic liquids: [5F-PhMeTAZ]+ or [5H-PhMeTAZ]+ which one is the better choice? J Fluorine Chem 257–258:109970. https://doi.org/10.1016/j.jfluchem.2022.109970
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789. https://doi.org/10.1103/PhysRevB.37.785
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor Chem Accounts 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
McLean AD, Chandler GS (1980) Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J Chem Phys 72:5639–5648. https://doi.org/10.1063/1.438980
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J Chem Phys 72:650–654. https://doi.org/10.1063/1.438955
Dunning TH Jr (1989) Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J Chem Phys 90:1007–1023. https://doi.org/10.1063/1.456153
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. J Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Grimme S, Antony J, Ehrlich S, Krieg H (2010) A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J Chem Phys 132:154104–154119. https://doi.org/10.1063/1.3382344
Levine IN (2013) Quantum chemistry, 7th edn. Pearson Education, London
Politzer P, Truhlar DG (2013) Chemical applications of atomic and molecular electrostatic potentials: reactivity, structure, scattering, and energetics of organic, inorganic, and biological systems. Springer, New York
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralta Jr JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, Revision A.02, Inc., Gaussian, Wallingford, CT
Lu T, Chen FW (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Biegler-Konig F, Schonbohm J, Bayles D (2001) AIM2000—a program to analyze and visualize atoms in molecules. J Comput Chem 22:545–560
Johnson ER, Keinan S, Mori-Sanchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Contreras-García J, Johnson ER, Keinan S, Chaudret R, Piquemal JP, Beratan DN, Yang W (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38. https://doi.org/10.1016/0263-7855(96)00018-5
Bader RFW (1990) Atoms in molecules: a quantum theory. Clarendon Press, Oxford
Schleyer PVR, Maerker C, Dransfeld A, Jiao H, Hommes NJRVE (1996) Nucleus-independent chemical shifts: a simple and efficient aromaticity probe. J Am Chem Soc 118:6317–6318. https://doi.org/10.1021/ja960582d
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:1873–1878. https://doi.org/10.1021/jp9805048
Wu P, Chaudret R, Hu X, Yang W (2013) Noncovalent interaction analysis in fluctuating environments. J Chem Theory Comput 9:2226–2234. https://doi.org/10.1021/ct4001087
Contreras-Garic J, Calatayud M, Piquemal JP, Recio JM (2012) Ionic interactions: comparative topological approach. Comput Theor Chem 998:193–201. https://doi.org/10.1016/j.comptc.2012.07.043
Acknowledgements
Support of this work from the Research Council of the University of Guilan is gratefully appreciated.
Author information
Authors and Affiliations
Contributions
BK contributed to conceptualization and writing—review and editing; KG contributed to formal analysis and methodology; NG and NP contributed to the investigation and provided the resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khalili, B., Ghauri, K., Ghavidel, N. et al. An insight into interaction of the uracil, thymine and cytosine biomolecules with methimazole anti-thyroid drug: DFT and GD3‑DFT approaches. Struct Chem 34, 1021–1042 (2023). https://doi.org/10.1007/s11224-022-02059-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02059-y