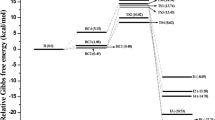
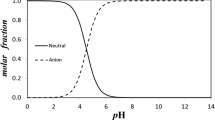
Abstract
The kinetics and mechanism in the oxidative degradation pathways of the •OH radical reaction with seven auxin mimic aromatic acid-based herbicides were investigated with the help of various theoretical methods. Various global and local reactivity parameters such as ionization energy, molecular hardness, electrophilicity, condensed Fukui function, and total energies were determined to predict the reactivity of these herbicides towards the •OH radical. Geometry optimization was performed at the CAM–B3LYP/6–311 + G(d) level of theory including the solvent effect using the polarizable continuum model (PCM) incorporating the integral equation formalism (IEF) with water as solvent. Single point energies of various species were calculated at ROMP2/aug–cc–pVDZ level of theory for better accuracy. The pKa values for these acid-based herbicides allow them to exist in the deprotonated form in aqueous condition. Hence, the calculations are also performed for the deprotonated form apart from the neutral species. The most reactive site for the •OH radical reaction is predicted and validated for neutral and deprotonated species. Once the most reactive site is known, the reaction rate constants are calculated theoretically by the traditional transition–state theory using one-dimensional tunneling corrections. The solvent effect on the reaction rate constant is implemented through Collins–Kimball formulations.





Similar content being viewed by others
Availability of data and materials
The various parameters of the molecular structure of the current study are available and can be obtained from the corresponding author on reasonable request.
References
Milne GWA (1995) CRC Handbook of Pesticides CRC Press, Taylor & Francis Group, Boca Raton, Florida, USA
Tu M, Hurd C, Randall JM (2001) Weed control methods handbook: tools & techniques for use in natural areas. The Nature Conservancy, All U.S. Government Documents (Utah Regional Depository). Paper 533
Gupta PK (2018) Toxicity of herbicides. In: Gupta RC (ed) Veterinary Toxicol. Elsevier Inc
Cobb A (1992:82–106) Auxin-type herbicides. Herbicides and Plant Physiol. Chapman Hall
Grossmann K (2007) Auxin herbicide action: lifting the veil step by step. Plant Signaling Behav 2:421–423
Todd OE, Figueiredo MRA, Morran S, Soni N, Preston C, Kubeš MF, Napier R, Gaines TA (2020) Synthetic auxin herbicides: finding the lock and key to weed resistance. Plant Sci 300
Tilman D, Cassman KG, Matson PA, Naylor R, Polasky S (2002) Agricultural sustainability and intensive production practices. Nature 418:671
Verma P, Verma P, Sagar R (2013) Variations in N mineralization and herbaceous species diversity due to sites, seasons, and N treatments in a seasonally dry tropical environment of India. For Ecol Manage 297:15–26
Xie H, Wang X, Chen J, Li X, Jia G, Zou Y, Zhang Y, Cui Y (2019) Occurrence, distribution and ecological risks of antibiotics and pesticides in coastal waters around Liaodong Peninsula. China Sci Total Environ 656:946–951
Donald DB, Cessna AJ, Sverko E, Glozier NE (2007) Pesticides in surface drinking-water supplies of the northern Great Plains. Environ Health Perspect 15:1183–1191
Rice PJ, Rice PJ, Arthur EL, Barefoot AC (2007) Advances in pesticide environmental fate and exposure assessments. J Agric Food Chem 55:5367–5376
Verma JP, Jaiswal DK, Sagar R (2014) Pesticide relevance and their microbial degradation: a-state-of-art. Rev Environ Sci Biotechnol 13:429–466
Tomco PL, Duddleston KN, Schultz EJ, Hagedorn B, Stevenson TJ, Seefeldt SS (2016) Field degradation of aminopyralid and clopyralid and microbial community response to application in Alaskan soils. Environ Toxicol Chem 35:485–493
De Lucas A, Rodriguez L, Villasenor J, Fernandez FJ (2007) Influence of industrial discharges on the performance and population of a biological nutrient removal process. Biochem Eng J 34:51–61
Fairchild JF, Feltz KP, Sappington LC, Allert AL, Nelson KJ, Valle J (2009) An ecological risk assessment of the acute and chronic toxicity of the herbicide picloram to the threatened bull trout (Salvelinus confluentus) and the rainbow trout (Onchorhyncus mykiss). Arch Environ Contam Toxicol 56:761–769
Garcia-Becerra FY, Ortiz I (2018) Biodegradation of emerging organic micropollutants in nonconventional biological wastewater treatment: a critical review. Environ Eng Sci 35:1012–1036
Gaur N, Narasimhulu K, PydiSetty Y (2018) Recent advances in the bioremediation of persistent organic pollutants and its effect on environment. J Clean Prod 198:1602–1631
Kumar S, Kaushik G, Dar MA, Nimesh S, Lopez-Chuken UJ, Villarreal-Chiu JF (2018) Microbial degradation of organophosphate pesticides: a review. Pedosphere 28:190–208
Ghanbari F, Moradi M (2017) Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: review. Chem Eng J 310:41–62
Solis RR, Rivas FJ, Gimeno O, Perez-Bote JL (2016) Photocatalytic ozonation of clopyralid, picloram and triclopyr. Kinetics, toxicity and influence of operational parameters. J Chem Technol Biotechnol 91:51–58
Šojić DV, Anderluh VB, Orčić DZ, Abramović BF (2009) Photodegradation of clopyralid in TiO2 suspensions: identification of intermediates and reaction pathways. J Hazard Mater 168:94–101
Rahman MA, Muneer M (2005) Heterogeneous photocatalytic degradation of picloram, dicamba, and floumeturon in aqueous suspensions of titanium dioxide. J Environ Sci Health Part B Pestic Contam Agric Wastes 40:247–267
Pinna MV, Pusino A (2012) Direct and indirect photolysis of two quinolinecarboxylic herbicides in aqueous systems. Chemosphere 86:655–658
Pareja L, Perez-Parada A, Aguera A, Cesio V, Heinzen H, Fernandez-Alba AR (2012) Photolytic and photocatalytic degradation of quinclorac inultrapure and paddy field water: identification of transformation products. Chemosphere 87:834–844
Djebbar K, Zertal A, Sehili T (2006) Photocatalytic degradation of 2,4- dichlorophenoxyacetic acid and 4- chloro-2-methylphenoxyacetic acid in water by using TiO2. Environ Technol 27:1191–1197
Huang C, Dong C, Tang Z (1993) Advanced chemical oxidation: its present role and potential future in hazardous waste treatment. Waste Manag 13:361–377
Semitsoglou-Tsiapou S, Templeton MR, Graham NJD, Leal LH, Martijn BJ, Royce A, Kruithof JC (2016) Low pressure UV/H2O2 treatment for the degradation of the pesticides metaldehyde, clopyralid and mecoprop-Kinetics and reaction product formation. Water Res 91:285–294
Sangami S, Manu B (2017) Optimization of Fenton’s oxidation of herbicide dicamba in water using response surface methodology. Appl Water Sci 7:4269–4280
Glaze WH (1987) Drinking-water treatment with ozone. Environ Sci Technol 21:224–230
Ahmad R, James TK, Rahman A, Holland PT (2003) Dissipation of the herbicide clopyralid in an allophanic soil: laboratory and field studies. J Environ Sci Health Part B Pestic Contam Agric Wastes 683–695
Elliott JA, Cessna AJ, Best KB, Nicholaichuk W, Tollefson LC (1998) Leaching and preferential flow of clopyralid under irrigation: field observations and simulation modeling. J Environ Qual 27:124–131
Tang L, Zeng GM, Shen GL, Li YP, Zhang Y, Huang DL (2008) Rapid detection of picloram in agricultural field samples using a disposable immunomembrane-based electrochemical sensor. Environ Sci Technol 42:1207–1212
Song X (2014) Dicamba. In: Wexler P (ed) Encyclopedia of toxicology (Third Edition). Elsevier Inc
Sanches-Neto FO, Ramos B, Lastre-Acosta AM, Teixeira ACSC, Carvalho-Silva VH (2021) Aqueous picloram degradation by hydroxyl radicals: unveiling mechanism, kinetics, and ecotoxicity through experimental and theoretical approaches. Chemosphere 278:130401
Ozcan A, Sahin Y, Koparal AS, Oturan MA (2008) Degradation of picloram by the electro-Fenton process. J Hazard Mater 153:718–727
Coledam DAC, Sanchez-Montes I, Silva BF, Aquino JM (2018) On the performance of HOCl/Fe2+, HOCl/Fe2+/UVA, and HOCl/UVC processes using in situ electrogenerated active chlorine to mineralize the herbicide picloram. Appl Catal B-Environ 227:170–177
Ghauch A (2001) Degradation of benomyl, picloram, and dicamba in a conical apparatus by zero-valent iron powder. Chemosphere 43:1109–1117
Contreras MBC, Fourcade F, Assadi A, Amrane A, Fernandez-Morales FJ (2019) Electro Fenton removal of clopyralid in soil washing effluents. Chemosphere 237:124447
Wu M-H, Yang X-Y, Xu G, Liu N, Guo R-Y, Lei J-Q, Tang L (2015) UV-based oxidation processes for removal of clopyralid: optimal conditions, efficiency, and by-products. Environ Eng Sci 32:998–1006
Tizaoui C, Mezughi K, Bickley R (2011) Heterogeneous photocatalytic removal of the herbicide clopyralid and its comparison with UV/H2O2 and ozone oxidation techniques. Desalination 273:197–204
Xu G, Liu N, Wu M, Bu T, Zheng M (2013) The photodegradation of clopyralid in aqueous solutions: effects of light sources and water constituents. Ind Eng Chem Res 52
Yang X, Cao X, Zhang L, Wu Y, Zhou L, Xiu G, Ferronato C, Chovelon J-M (2021) Sulfate radical-based oxidation of the aminopyralid and picloram herbicides: the role of amino group on pyridine ring. J Hazard Mater 405:124181
Yang X, Ding X, Zhou L, Fan H-h, Wang X, Ferronato C, Chovelon J-M, Xiu G (2020) New insights into clopyralid degradation by sulfate radical: pyridine ring cleavage pathways. Water Res 171:115378
Zhu J, Ding G, Liu Y, Wang B, Zhang W, Guo M, Geng Q, Cao Y (2015) Ionic liquid forms of clopyralid with increased efficacy against weeds and reduced leaching from soils. Chem Eng J 279:472–477
Thiam A, Sirés I, Salazar R, Brillas E (2018) On the performance of electrocatalytic anodes for photoelectro-Fenton treatment of synthetic solutions and real water spiked with the herbicide chloramben. J Environ Manage 224:340–349
Guin M, Nayak AN, Gowda NMM (2016) Theoretical study of hydrogen bonded picolinic acid-water complexes. Indian J Chem 55:782–792
Domingo LR, Pérez P, Sáez JA (2013) Understanding the local reactivity in polar organic reactions through electrophilic and nucleophilic Parr functions. RSC Adv 3:1486–1494
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange−correlation functional using the coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Parr RG, Yang W (1982) Density functional theory for atoms and molecules. Oxford University Press, New York
Pearson RG (1997) Chemical hardness. John Wiley-VCH, Weinheim
Pearson RG (2005) Chemical hardness and density functional theory. J Chem Sci 117:369–377
Chandrakumar KRS, Pal S (2002) The concept of density functional theory based descriptors and its relation with the reactivity of molecular systems: a semi-quantitative study. Int J Mol Sci 3:324–337
Sánchez-Márquez J (2019) Correlations between Fukui indices and reactivity descriptors based on Sanderson’s principle. J Phys Chem A 123:8571–8582
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su S et al (1993) General atomic and molecular electronic structure system. J Comput Chem 14:1347−1363
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA et al (2010) Gaussian 09, revision B.01. In: Gaussian, Inc., Wallingford, CT
Carvalho-Silva VH, Aquilanti V, de Oliveira HCB, Mundim KC (2017) Deformed transition-state theory: deviation from Arrhenius behavior and application to bimolecular hydrogen transfer reaction rates in the tunneling regime. J Comput Chem 38:178–188
Collins FC, Kimball GE (1949) Diffusion-controlled reaction rates. J Colloid Sci 4:425–437
Machado HG, Sanches-Neto FO, Coutinho ND, Mundim KC, Palazzetti F, Carvalho-Silva VH (2019) “Transitivity”: a code for computing kinetic and related parameters in chemical transformations and transport phenomena. Molecules 24:3478
Dzib E, Cabellos JL, Ortíz-Chi F, Pan S, Galano A, Merino G (2019) Eyringpy: a program for computing rate constants in the gas phase and in solution. Int J Quantum Chem 119:e25686
McKinney JD, Richard A, Waller C, Newman MC, Gerberick F (2000) The practice of structure activity relationships (SAR) in toxicology. Toxicol Sci 56:8–17
Mayo-Bean K, Moran-Bruce K, Meylan W, Ranslow P, Lock M, Nabholz JV, Runnen JV, Cassidy LM, Tunkel J (2017) Estimating toxicity of industrial chemicals to aquatic organisms using the ECOSAR (ECOlogical Structure-Activity Relationship) class program Version 2.0. U.S. Environ Protection Agency
Acknowledgements
The author thanks Prof. G. Naresh Patwari for some of the calculations performed at IITBombay, Mumbai.
Funding
The work was supported by DAE under Project “26–XII–CR& D–117.03/Advanced Research on Laser–Induced Chemical Reactions.”
Author information
Authors and Affiliations
Contributions
As a single-author manuscript, starting from the conceptualization to theoretical calculation, writing, presentation, and figures, everything was handled by the author. For the remaining, it has been duly acknowledged.
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Upadhyaya, H.P. Theoretical studies on the mechanism, kinetics, and degradation pathways of auxin mimic herbicides by •OH radical in aqueous media. Struct Chem 34, 931–943 (2023). https://doi.org/10.1007/s11224-022-02055-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02055-2