Abstract
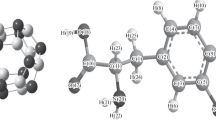
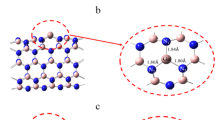
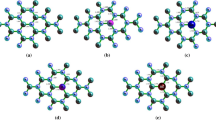
The adsorption behavior of the anti-neurodegenerative drug Levodopa (LD) on pristine and aluminum-doped (Al-doped) boron nitride nanotubes (BNNTs) has been investigated in the current study using the density functional theory (DFT) approach at the B3LYP/6-31G** level of theory. The aim was to improve and expand boron nitride nanotubes drug carriers used in biomedical systems, i.e., drug delivery systems. The binding qualities of pure and doped BNNT complexes as adsorbents with LD were explored using the natural bond orbitals (NBO) analysis, density of state (DOS), electrical and structural characteristics, and atoms in molecules (AIM) properties. Due to doping heteroatoms in the adsorbent's molecular structure, the obtained data reveal a gradual shift in LD adsorption, with a significant rise in negative adsorption energy values.
The electronic perturbation caused by doped atoms, particularly Al, improves boron nitride nanotube sensitivity to adsorbed Levodopa, and the electronic properties of the nanotubes are altered following Levodopa adsorption in the complex. As the frontier molecular orbital distributions were transferred from LD to BNNTs in the complex of BNNT–LD, it was also shown that LD drugs could be loaded on pristine and Al-doped BNNTs while remaining safe from interactions with other substances. Furthermore, AIM analysis based investigations revealed that O–Al interaction in LD adsorbed on Al-doped boron nitride nanotube and O–N interaction in the BNNT–LD complex are partially covalent.
Finally, the findings showed that pristine and Al-doped BNNT could be used in drug delivery processes by controlling the loaded LD contribution to future interactions.






Similar content being viewed by others
Data availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable
References
Iijima S, Ichihashi T (1993) Single-shell carbon nanotubes of 1-nm diameter. Nature 363:603–605. https://doi.org/10.1038/363603a0
Felegari Z, Hamedani S (2022) Adsorption properties and quantum molecular descriptors of the anticancer drug cytophosphane on the armchair single-walled carbon nanotubes: a DFT study. Lett Org Chem 19:1034–1041. https://doi.org/10.2174/1570178619666220509095156
Chopra NG, Luyken RJ, Cherrey K, Crespi VH, Cohen ML, Louie SG, Zettl A (1995) Boron nitride nanotubes. Science 269:966–967. https://doi.org/10.1126/science.269.5226.966
Rubio A, Corkill JL, Cohen ML (1994) Theory of graphitic boron nitride nanotubes. Phys Rev B 49:5081. https://doi.org/10.1103/PhysRevB.49.5081
Han WQ, Mickelson W, Cumings J, Zettl A (2002) Transformation of B x C y N z nanotubes to pure BN nanotubes. Appl Phys Lett 81:1110–1112. https://doi.org/10.1063/1.1498494
Golberg D, Bando Y, Tang CC, Zhi CY (2007) Boron nitride nanotubes. Adv Mater 19:2413–2432. https://doi.org/10.1002/adma.200700179
Blase X, Rubio A, Louie SG, Cohen ML (1994) Stability and band gap constancy of boron nitride nanotubes. EPL (Europhysics Letters) 28:335. https://doi.org/10.1209/0295-5075/28/5/007
Golberg D, Bando Y, Kurashima K, Sato T (2001) Synthesis and characterization of ropes made of BN multiwalled nanotubes. Scripta Mater 44:1561–1565. https://doi.org/10.1016/S1359-6462%2801%2900724-2
Nakhmanson SM, Calzolari A, Meunier V, Bernholc J, Nardelli MB (2003) Spontaneous polarization and piezoelectricity in boron nitride nanotubes. Phys Rev B 67:235406. https://doi.org/10.1103/PhysRevB.67.235406
Liew KM, Yuan J (2011) High-temperature thermal stability and axial compressive properties of a coaxial carbon nanotube inside a boron nitride nanotube. Nanotechnology 22:085701. https://doi.org/10.1088/0957-4484/22/8/085701
Chang CW, Fennimore AM, Afanasiev A, Okawa D, Ikuno T, Garcia H, Li D, Majumdar A, Zettl A (2006) Isotope effect on the thermal conductivity of boron nitride nanotubes. Phys Rev Lett 97:085901. https://doi.org/10.1103/PhysRevLett.97.085901
Weng Q, Wang B, Wang X, Hanagata N, Li X, Liu D, Wang X, Jiang X, Bando Y, Golberg D (2014) Highly water-soluble, porous, and biocompatible boron nitrides for anticancer drug delivery. ACS Nano 8:6123–6130. https://doi.org/10.1021/nn5014808
Ciofani G, Danti S, Nitti S, Mazzolai B, Mattoli V, Giorgi M (2013) Biocompatibility of boron nitride nanotubes: an up-date of in vivo toxicological investigation. Int J Pharm 444:85–88. https://doi.org/10.1016/j.ijpharm.2013.01.037
Bououden W, Benguerba Y, Darwish AS, Attoui A, Lemaoui T, Balsamo M, Erto A, Alnashef IM (2021) Surface adsorption of Crizotinib on carbon and boron nitride nanotubes as Anti-Cancer drug Carriers: COSMO-RS and DFT molecular insights. J Mol Liq 338:116666. https://doi.org/10.1016/j.molliq.2021.116666
Yuksel N, Kose A, Fellah MF (2022) A density functional theory study for adsorption and sensing of 5-Fluorouracil on Ni-doped boron nitride nanotube. Mater Sci Semicond Process 137:106183. https://doi.org/10.1016/j.mssp.2021.106183
Poorsargol M, Setayesh-Mehr Z (2022) Betulinic acid and 3-o-acetyl-betulinic acid interactions with external and internal surface of boron-nitride nanotubes: a DFT and MD investigation. Computatio Theoretical Chem 113738. https://doi.org/10.1016/j.comptc.2022.113738
Nafiu S, Apalangya VA, Yaya A, Sabi EB (2022) Boron nitride nanotubes for curcumin delivery as an anticancer drug: a DFT investigation. Appl Sci 12:879. https://doi.org/10.3390/app12020879
Azarakhshi F, Shahab S, Kaviani S, Sheikhi M (2021) Investigation of adsorption of sulfanilamide drug on surfaces of the B12N12 and Al12N12 fullerenes: a DFT study. Lett Org Chem 18:640–655. https://doi.org/10.2174/1570178617999201013170019
Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F (2010) Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging 5:229. https://doi.org/10.2147/CIA.S6456
Olanow CW, Obeso JA, Stocchi F (2006) Continuous dopamine-receptor treatment of Parkinson’s disease: scientific rationale and clinical implications. The Lancet Neurology 5:677–687. https://doi.org/10.1016/S1474-4422(06)70521-X
Whone AL, Watts RL, Stoessl AJ, Davis M, Reske S, Nahmias C, Lang AE, Rascol O, Ribeiro MJ, Remy P, Poewe WH (2003) Slower progression of Parkinson’s disease with ropinirole versus Levodopa: the REAL-PET study. Ann Neurol 54:93–101. https://doi.org/10.1002/ana.10609
Olanow CW, Agid Y, Mizuno Y, Albanese A, Bonucelli U, Damier P, De Yebenes J, Gershanik O, Guttman M, Grandas F, Hallett M (2004) Levodopa in the treatment of Parkinson’s disease: current controversies. Mov Disord 19:997–1005. https://doi.org/10.1002/mds.20243
Soltani A, Raz SG, Rezaei VJ, Khalaji AD, Savar M (2012) Ab initio investigation of Al-and Ga-doped single-walled boron nitride nanotubes as ammonia sensor. Appl Surf Sci 263:619–625. https://doi.org/10.1016/j.apsusc.2012.09.122
Muniyandi S, Sundaram R, Kar T (2018) Aluminum doping makes boron nitride nanotubes (BNNTs) an attractive adsorbent of hydrazine (N2H4). Struct Chem 29:375–382. https://doi.org/10.1007/s11224-017-1034-8
Ahmadi Peyghan A, Hadipour NL, Bagheri Z (2013) Effects of Al doping and double-antisite defect on the adsorption of HCN on a BC2N nanotube: density functional theory studies. J Phys Chem C 117:2427–2432. https://doi.org/10.1021/jp312503h
Heidari H, Afshari S, Habibi E (2015) Sensing properties of pristine, Al-doped, and defected boron nitride nanosheet toward mercaptans: a first-principles study. RSC Adv 5:94201–94209. https://doi.org/10.1039/C5RA09923D
Azizi K, Karimpanah M (2013) Computational study of Al-or P-doped single-walled carbon nanotubes as NH3 and NO2 sensors. Appl Surf Sci 15:102–109. https://doi.org/10.1016/j.apsusc.2013.07.146
Bader R (1990) Atoms in molecules: a quantum theory: Oxford Univ. Press, Oxford
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1998) NBO 3.1, TCI; University of Wisconsin, Madison, WI
Becke AD (1996) Density-functional thermochemistry. IV. A new dynamical correlation functional and implications for exact-exchange mixing. J Chem Phys 104:1040–1046. https://doi.org/10.1063/1.470829
Lee C, Yang W, Parr RG (1988) Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785. https://doi.org/10.1103/PhysRevB.37.785
Miehlich B, Savin A, Stoll H, Preuss H (1989) Results obtained with the correlation energy density functionals of Becke and Lee, Yang and Parr. Chem Phys Lett 157:200–206. https://doi.org/10.1016/0009-2614(89)87234-3
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H (2009) Gaussian 09; Gaussian, Inc. Wallingford, CT 32:5648–5652
Frisch A, Hratchian HP, Dennington RD, Keith TA, Millam J, Nielsen AB, Holder AJ, Hiscocks J (2009) Gaussian., Inc. GaussView Version 5.0. 8
O'boyle NM, Tenderholt AL, Langner KM (2008) Cclib: a library for package‐independent computational chemistry algorithms. J Comput Chem 29:839-845.https://doi.org/10.1002/jcc.20823
Mulliken RS (1934) A new electroaffinity scale; together with data on valence states and on valence ionization potentials and electron affinities. J Chem Phys 2:782–793. https://doi.org/10.1063/1.1749394
Parr RG, Yang W (1989) Density functional theory of atoms and molecules. Oxford University Press:3–5
Parr RG, Pearson RG (1983) Absolute hardness: companion parameter to absolute electronegativity. J Am Chem Soc 105:7512–7516. https://doi.org/10.1021/ja00364a005
Chattaraj PK, Roy DR (2007) Update 1 of: electrophilicity index. Chem Rev 107:46–74. https://doi.org/10.1021/cr078014b
König FB, Schönbohm J, Bayles D (2001) AIM2000-a program to analyze and visualize atoms in molecules. J Comput Chem 22:545–559
Bader RF (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323. https://doi.org/10.1021/jp981794v
Hendriks KH, Li W, Wienk MM, Janssen RA (2014) Small-bandgap semiconducting polymers with high near-infrared photoresponse. J Am Chem Soc 136:12130–12136. https://doi.org/10.1021/ja506265h
Badenhoop JK, Weinhold F (1999) Natural steric analysis of internal rotation barriers. Int J Quantum Chem 72:269–280. https://doi.org/10.1002/(SICI)1097-461X(1999)72:4%3C269::AID-QUA9%3E3.0.CO;2-8
Hamedani S, Hamedani E (2017) Boron nitride nanotubes as novel vectors for drug delivery of amino acids: a first principles simulation. Chinese J Structural Chem 36:1562–1567. https://doi.org/10.14102/j.cnki.0254-5861.2011-1552
Keith TA, Bader RF, Aray Y (1996) Structural homeomorphism between the electron density and the virial field. Int J Quantum Chem 57:183–198. https://doi.org/10.1002/(SICI)1097-461X(1996)57:2%3C183::AID-QUA4%3E3.0.CO;2-U
Author information
Authors and Affiliations
Contributions
Conception and design of study: Shahla Hamedani and Melina Shadi. Acquisition of data: Melina Shadi. Analysis and/or interpretation of data: Melina Shadi and Shahla Hamedani. Drafting the manuscript: Melina Shadi. Revising the manuscript critically for important intellectual content: Melina Shadi and Shahla Hamedani. Approval of the version of the manuscript to be published: Melina Shadi and Shahla Hamedani. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Shadi, M., Hamedani, S. A DFT approach to the adsorption of the Levodopa anti-neurodegenerative drug on pristine and Al-doped boron nitride nanotubes as a drug delivery vehicle. Struct Chem 34, 905–914 (2023). https://doi.org/10.1007/s11224-022-02050-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02050-7