Abstract
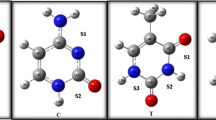
The use of drug combinations can be useful in the treatment of various cancers. In this work, we studied the interaction of tegafur (TG) drug with uracil (U), 5-fluorouracil (FU), and gimeracil (GI) as anti-tumor drugs. The DFT calculations were carried out to determine the structural parameters, interaction energies, strength of interactions, physical and topological properties of the TG/U, TG/FU, and TG/GI complexes. The chemical reactivity of U, FU, and GI molecules to TG was investigated using the molecular quantum descriptors. From the results, intermolecular interactions in complexes are of the hydrogen bonding type. The Espinosa-Molins-Lecomte (EML) equation was used to calculate the energy of hydrogen bonds. The effect of binding sites on the strength of hydrogen bonds and the stability of complexes was investigated. To evaluate the electronic transfers in complexes, the natural bond orbital (NBO) analysis was performed. The strength and nature of interactions were determined by atoms in molecules (AIM) and reduced density gradient (RDG) analysis.







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author, upon reasonable request.
References
Freddie B, Mathieu L, Elisabete W, Isabelle S (2021) The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 127:2864–2866. https://doi.org/10.1002/cncr.33587
DeVita VT, Chu E (2008) A history of cancer chemotherapy. Cancer Res 68:8643–8653. https://doi.org/10.1158/0008-5472.CAN-07-6611
Heidelberger C, Chaudhuri NK, Danneburg P, Mooren D, Griesbach L (1957) Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature 179:663–666. https://doi.org/10.1038/179663a0
Reni M, Cereda S, Galli L (2007) PEFG (cisplatin, epirubicin, 5-fluorouracil, gemcitabine) for patients with advanced pancreatic cancer: the ghost regimen. Cancer Let 256:25–28. https://doi.org/10.1016/j.canlet.2007.04.009
Wanga W, Collie-Duguida E, Cassidy J (2002) Cerivastatin enhances the cytotoxicity of 5-fluorouracil on chemosensitive and resistant colorectal cancer cell lines. FEBS Let 531:415–420. https://doi.org/10.1016/S0014-5793(02)03575-5
Lutterbeck CA, Wilde ML, Baginska E, Leder C, Machado EL, Kümmerer K (2016) Degradation of cyclophosphamide and 5-fluorouracil by UV and simulated sunlight treatments: assessment of the enhancement of the biodegradability and toxicity. Environmen Pollut 208:467–476. https://doi.org/10.1016/j.envpol.2015.10.016
Prasad O, Sinha L, Kumar N (2010) Theoretical Raman and IR spectra of tegafur and comparison of molecular electrostatic potential surfaces, polarizability and hyerpolarizability of tegafur with 5-fluoro-uracil by density functional theory. Journal of Atomic and Molecular Sciences 1:201–214. https://doi.org/10.4208/jams.032510.042010a
Arias JL, López-Viota M, Gallardo V, Ruiz MA (2010) Chitosan nanoparticles as a new delivery system for the chemotherapy agent tegafur. Drug Dev Ind Pharm 36:744–750. https://doi.org/10.3109/03639040903517914
Li VS, Choi D, Wang Z, Jimenez LS, Tang MS, Kohn H (1996) Role of the C-10 substituent in mitomycin C-1−DNA bonding. J Am Chem Soc 118:2326–2331. https://doi.org/10.1021/ja953871v
Tomasz M, Lipman R, Chowdary D, Pawlak J, Verdine GL, Nakanishi K (1987) Isolation and structure of a covalent cross-link adduct between mitomycin C and DNA. Science 235:1204–1208. https://doi.org/10.1126/science.3103215
Sakata K, Someya M, Matsumoto Y, Tauchi H, Kai M, Toyota M, Takagi M, Hareyama M, Fukushima M (2011) Gimeracil an inhibitor of dihydropyrimidine dehydrogenase, inhibits the early step in homologous recombination. Cancer Sci 102:1712–1716. https://doi.org/10.1111/j.1349-7006.2011.02004.x
Lu DY (2015) Drug combinations, personalized cancer chemotherapy, Book Chapter, pp 37–41
Makiabadi B, Kian H (2015) The hydrogen bond interactions in glycine-nitrosamine complexes: a DFT study. Monatshefte fur Chemie 146:69–78. https://doi.org/10.1007/s00706-014-1304-8
Makiabadi B, Zakarianezhad M (2020) Investigation of adsorption of the nitrosamine molecule as a carcinogen agent on the AlN nanotubes: a DFT study. Chemical Methodologies 4:191–202. https://doi.org/10.33945/SAMI/CHEMM.2020.2.9
Zakarianezhad M, Habibi-Khorassani SM, Maghsoodlou MT, Makiabadi B (2012) Understanding the mechanism of stable phosphorus ylides derived from imidazole, 2-Methylimidazole or 4-Methylimidazole: a kinetic study. Orient J Chem 28:1259–1269. https://doi.org/10.13005/ojc/280322
Masoodi HR, Zakarianezhad M, Bagheri S, Makiabadi B, Shool M (2014) Substituent effects on some calculated NMR data in T-shaped configuration of benzene dimer. Chem Phys Lett 614:143–147. https://doi.org/10.1016/j.cplett.2014.09.021
Kobayakawa M, Kojima Y (2011) Tegafur/gimeracil/oteracil (S-1) approved for the treatment of advanced gastric cancer in adults when given in combination with cisplatin: a review comparing it with other fluoropyrimidine-based therapies. Onco Targets Ther 4:193–201. https://doi.org/10.2147/OTT.S19059
Zhang E, Fu A, Chen W, Wang X (2018) Tumor suppression by Tegafur combined with Barbadian in S-180 tumor-bearing mice. Oncol Lett 16:5673–5678. https://doi.org/10.3892/ol.2018.9373
Peng M, Ding Y, Yu L, Deng Y, Lai W, Hu Y, Zhang H, Wu X, Fan H, Ding H, Wu Y, Tao G (2015) Tegafur substitution for 5-Fu in combination with actinomycin D to treat gestational trophoblastic. PLoS ONE 10:1–9. https://doi.org/10.1371/journal.pone.0138146
Lee BR, Yu JY, Yoon SH, Ban HJ, Kwon YS, Oh IJ, Kim KS, Kim YI (2015) Lim S-Ch, Kim Y. Ch, Variability in the anti-tumor effect of tegafur-uracil depending on histologic types of lung cancer, J Thorac Dis 7:433–438. https://doi.org/10.3978/j.issn.2072-1439.2015.01.22
Kashiwabara K, Semba H, Fujii S, Tsumura S (2018) Toxicity and efficacy of sequential chemotherapy in patients with p-stage I non-small cell lung cancer that recurring during postoperative tegafur-uracil adjuvant chemotherapy. Cancer Invest 36:424–430. https://doi.org/10.1080/07357907.2018.1515954
Matsuda C, Ishiguro M, Teramukai S, Kajiwara Y, Fujii S, Kinugasa Y, Nakamoto Y, Kotake M, Sakamoto Y, Kurachi K, Maeda A, Komori K, Tomita N, Shimada Y, Takahashi K, Kotake K, Watanabe M, Mochizuki H, Nakagawa Y, Sugihara K (2018) A randomised-controlled trial of 1-year adjuvant chemotherapy with oral tegafur-uracil versus surgery alone in stage II colon cancer: SACURA trial. Eur J Cancer 96:54–63. https://doi.org/10.1016/j.ejca.2018.03.009
Wellington K, Goa K (2001) Oral tegafur/uracil. Drugs Aging 18:935–948. https://doi.org/10.2165/00002512-200118120-00005
Hsieh MY, Chen MK (2018) ASO author reflections: tegafur–uracil in advanced oral cancer. Ann Surg Oncol 25:899–900. https://doi.org/10.1245/s10434-018-6952-1
Meropol NJ (1998) Oral fluoropyrimidines in the treatment of colorectal cancer. Eur J Cancer 34:1509–1513. https://doi.org/10.1016/S0959-8049(98)00226-3
Yeh YS, Tsai HL, Huang ChW, Wang JY (2017) Maintenance tegafur-uracil versus observation following an adjuvant oxaliplatin-based regimen in patients with stage III colon cancer after radical resection: study protocol for a randomized controlled trial. Trials 18:191–203. https://doi.org/10.1186/s13063-017-1904-9
Kajita J, Fuse E, Kuwabara T, Kobayashi H (2003) The contribution of cytochrome P450 to the metabolism of tegafur in human liver. Drug Metab Pharmacokinet 18:303–309. https://doi.org/10.2133/dmpk.18.303
Labianca R, Beretta GD, Mosconi S, Milesi L (2004) The role of uracil-tegafur (UFT) in elderly patients with colorectal cancer. Crit Rev Oncol Hematol 52:73–80. https://doi.org/10.1016/j.critrevonc.2004.06.001
Shirasaka T, Nakano K, Takechi T, Satake H, Uchida J, Fujioka A, Saito H, Okabe H, Oyama K, Takeda S, Unemi N, Fukushima M (1996) Antitumor activity of 1 M tegafur-0.4 M 5-chloro-2,4-dihydroxypyridine-1 M potassium oxonate (S-1) against human colon carcinoma orthotopically implanted into nude rats. Cancer Res 56:2602–2906
Tanaka F, Wada H, Fukushima M (2010) UFT and S-1 for treatment of primary lung cancer. Gen Thorac Cardiovasc Surg 58:3–13. https://doi.org/10.1007/s11748-009-0498-x
Malet-Martino M, Martino R (2002) Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. Oncologist 7:288–323. https://doi.org/10.1634/theoncologist.7-4-288
Hoff PM, Pazdur R (1998) UFT plus oral leucovorin: a new oral treatment for colorectal cancer. Oncologist 3:155–164. https://doi.org/10.1634/theoncologist.3-3-155
Hortobagyi GN, Young RR, Karwal M, Ibrahim NK, Hermann R, James L, Murray JS, Watkins SP, Jr-Ira G (2010) A phase 2 study of a fixed combination of uracil and ftorafur and leucovorin given orally in a twice-daily regimen to treat patients with recurrent metastatic breast cancer. Cancer 116:2301–2306. https://doi.org/10.1002/cncr.24900
Matt P, Bv Z-B, Rojas GC, Hofstede H, Garcia-Carbonero R, Camarero J, Abadie E, Pignatti F (2011) The European Medicines Agency review of tegafur/gimeracil/oteracil (Teysuno™) for the treatment of advanced gastric cancer when given in combination with cisplatin: summary of the scientific assessment of the Committee for Medicinal Products for Human Use (CHMP). Oncologist 16:1451–1457. https://doi.org/10.1634/theoncologist.2011-0224
Braga SF, Melo LC, Barone PMVB (2004) Semiempirical study on the electronic structure of antitumor drugs ellipticines, olivacines and isoellipticines. J Mol Struct Theochem 10:51–59. https://doi.org/10.1016/j.theochem.2004.07.039
Nishimura T, Okobira T, M. Kelly A, Shimada N, Takeda Y, Sakurai K (2007) DNA binding of tilorone:1H NMR and calorimetric studies of the intercalation. J Biochem 46:8156–8163. https://doi.org/10.1021/bi602402m
Makiabadi B, Zakarianezhad M, Hosseini SS (2021) Investigation and comparison of pristine/doped BN, AlN, and CN nanotubes as drug delivery systems for Tegafur drug: a theoretical study. Struct Chem 32:1019–1037. https://doi.org/10.1007/s11224-020-01680-z
Khanmohammadi A, Mohammadi M (2019) Theoreticalstudy of various solvents effect on 5-fluorouracil-vitamin B3 complex using PCM method. J Chil Chem Soc 64:4337–4344. https://doi.org/10.4067/s0717-97072019000104337
Frisch M, Trucks G, Schlegel HB, Scuseria G, Robb M, Cheeseman J, Scalmani G, Barone V, Mennucci B, Petersson G (2009) Gaussian 09, Revision A. 02, Gaussian. Inc, Wallingford, CT
Miertus S, Scrocco E (1981) Electrostatic interaction of a solute with a continuum. A direct utilizaion of AB initio molecular potentials for the prevision of solvent effects. J Chem Phys 55:117–129. https://doi.org/10.1016/0301-0104(81)85090-2
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926. https://doi.org/10.1021/cr00088a005
Biegler-König F, Schönbohm J, Bayles D (2001) AIM2000 —a program to analyze and visualize atoms in molecules. J Comput Chem 22:545–559. https://doi.org/10.1002/1096-987X(20010415)22:5%3c545:AID-JCC1027%3e3.0.CO;2-Y
Lu T, Chen F (2012) Multiwfn: a multifunctional wavefunction analyzer. J Comput Chem 33:580–592. https://doi.org/10.1002/jcc.22885
Kurinovich MA, Lee JK (2002) The acidity of uracil from the gas phase to solution: the coalescence of the N1 and N3 sites and implications for biological glycosylation. J Am Chem Soc 122:6258–6262. https://doi.org/10.1021/ja000549y
Whittleton SR, Hunter KC, Wetmore SD (2004) Effects of hydrogen bonding on the acidity of uracil derivatives. J Phys Chem A 108:7709–7718. https://doi.org/10.1021/jp048318r
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173. https://doi.org/10.1016/S0009-2614(98)00036-0
Jun-ichi A (1999) Reduced HOMO-LUMO Gap as an index of kinetic stability for polycyclic aromatic hydrocarbons. Phys Chem A 103:7487–7495. https://doi.org/10.1021/jp990092i
Johnson ER, Keinan S, Mori-Sánchez P, Contreras-García J, Cohen AJ, Yang W (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w.y
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by BM, MZ, and EZ. The first draft of the manuscript was written by BM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval
Not applicable
Consent to participate
All authors consent to participate in the research project and the following has been explained to us: the research may not be of direct benefit to us. my participation is completely voluntary.
Consent for publication
(a) Neither the article nor portions of it have been previously published elsewhere (except as an abstract or as part of a dissertation) (b) The manuscript is not under consideration for publication in another journal and will not be submitted elsewhere until the ELA editorial process is completed. (c) All authors consent to the publication of the manuscript in ELA should the article be accepted by the Editor-in-chief upon completion of the refereeing process.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Makiabadi, B., Zakarianezhad, M. & Zeydabadi, E. The role of hydrogen bonds on the stability of anticancer drug compounds TG/uracil, TG/5-fluorouracil and TG/gimeracil. Struct Chem 34, 755–767 (2023). https://doi.org/10.1007/s11224-022-02028-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-02028-5