Abstract
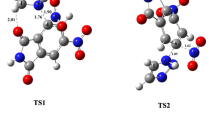
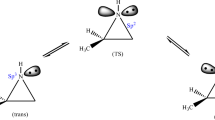
The mechanistic pathways for nucleophilic aromatic substitution of the nitro groups of 5,7-dinitro-3H-quinazolin-4-one and 1,3-dinitro-10H-dibenzo[b,f][1,4]oxazepin-11-one with 1H-1,2,3-triazole have been studied in both gas and solvent phase employing DFT/B3LYP calculations using 6-31G(d,p) basis set. The energetic parameters, i.e., free energy profiles along the reaction route and H-bond energy, charge analysis, and structural parameters have supported mechanistic pathway involving one-step concerted mechanism via formation of transition state during the reaction course. Despite substantial steric hindrance at adjacent position to fusion point, the regioselective attack of 1H-1,2,3-triazole has been observed at peri-position of benzannulated heterocycles. This regioselectivity is credited to intramolecular hydrogen bond C–H···O = C which stabilizes peri-transition state formation rather than para-transition state. The hydrogen bond acceptor–donor interactions have been studied using AIM method. The DFT and AIM analysis-derived results are compared for different sized dinitro-substituted benzannulated heterocycles and these studies have confirmed the contribution of substantial steric hindrance at peri-position with increased size of heterocycle. As benzannulated heterocycles are quite significant from the perspective of medicinal and natural product chemistry, this work will fulfill the need to find an efficient synthetic method for benzannulated heterocycles.












Similar content being viewed by others
Data availability
Supplementary data is provided with the submission.
References
Evans PA, Holmes B (1991) Medium ring nitrogen heterocycles. Tetrahedron 47(44):9131–9166
Cao D, Zhan K, An R, Xu H, Hao S, Yang X, Hou Z, Guo C (2019) The efficient synthesis of benzannulated seven-membered O-heterocycles via the intramolecular ring-opening cyclization of cyclopropanes. Org Lett 21(22):8948–8951
Ouyang X, Tamayo N, Kiselyov AS (1999) Solid support synthesis of 2-substituted dibenz[b, f] oxazepin-11(10H)-ones via SNAr methodology on AMEBA resin. Tetrahedron 55(10):2827–2834
Katritzky AR, Ramsden CA, Joule JA, Zhdankin VV (2010) Handbook of heterocyclic chemistry. Elsevier
Yuan L, Huang W, Gao Y, Gao X, Hu Q, Ma Y (2014) A new chromone from the stems of Cassia fistula and its anti-tobacco mosaic virus activity. Chem Asian J 26(23):8253
Kotha S, Mandal K, Tiwari A, Mobin SM (2006) Diversity-oriented approach to biologically relevant molecular frameworks starting with β-naphthol and using the Claisen rearrangement and olefin metathesis as key steps. Chem Eur J 12(31):8024–8038
Hino K, Nakamura H, Kato S, Irie A, Nagai Y, Uno H (1988) Nonsteroidal anti-inflammatory agents III synthesis of the metabolites of 10, 11-dihydro-8, α-dimethyl-11-oxodibenz [b, f] oxepin-2-acetic acid (bermoprofen). Chem Pharm Bull 36(9):3462–3467
Nakamura H, Yokoyama Y, Seto Y, Kadokawa T, Shimizu M (1984) AD-1590, a potent antagonist of lipopolysaccharide-induced fever in rabbits. J Pharm Pharmacol 36(3):182–186
Asai T, Otsuki S, Sakurai H, Yamashita K, Ozeki T, Oshima Y (2013) Benzophenones from an endophytic fungus, Graphiopsis chlorocephala, from Paeonia lactiflora cultivated in the presence of an NAD+-dependent HDAC inhibitor. Org Lett 15(8):2058–2061
Lin J, Liu S, Sun B, Niu S, Li E, Liu X, Che Y (2010) Polyketides from the ascomycete fungus Leptosphaeria sp. J Nat Prod 73(5):905–910
Kislyi KA, Samet AV, Strelenko YA, Semenov VV (2008) Synthetic utilization of polynitroaromatic compounds. Remarkable regioselectivity in nucleophilic displacement of aromatic nitro groups with amines. J Org Chem 73(6):2285–2291
Fischer W, Kvita V (1985) Aromatic nucleophilic substitution. Part 2. Preparation of novel 3‐substituted xanthone and thioxanthone derivatives. Helv Chim Acta 68(4):854–859
Samet AV, Marshalkin VN, Kislyi KA, Chernysheva NB, Strelenko YA, Semenov VV (2005) Synthetic utilization of polynitroaromatic compounds. Preparation of substituted dibenz [b, f][1, 4] oxazepine-11 (10 H)-ones from 2, 4, 6-trinitrobenzoic acid via nucleophilic displacement of nitro groups. J Org Chem 70(23):9371–9376
Samet AV, Marshalkin VN, Lyssenko KA, Semenov VV (2009) Synthesis of substituted dibenz [b, f] oxepines from 2, 4, 6-trinitrotoluene. Russ Chem Bull 2(58):347–350
Singh A, Singh A, A, Singh K, Singh G, Saroa A, (2021) Role of non-conventional hydrogen bonding in controlling regioselectivity for nucleophilic aromatic substitution of 4, 6-dinitroisoindoline-1, 3-dione with 1, 2, 3-triazole isomers: a computational studies. Struct Chem 32(3):1269–1278
Turi L, Dannenberg JJ (1993) Molecular orbital studies of CH... O hydrogen-bonded complexes. J Phys Chem A 97(30):7899–7909
Steiner T, Lutz B, van der Maas J, Schreurs AM, Kroon J, Tamm M (1998) Very long C-H··· O contacts can be weak hydrogen bonds: experimental evidence from crystalline [Cr (CO) 3 {η 6-[7-exo-(C [triple bond, length half m-dash] CH) C7 H7]}]. Chem Commun 2:171–172
Seiler P, Weisman GR, Glendening ED, Weinhold F, Johnson VB, Dunitz JD (1987) Observation of an eclipsed C-CH3 bond in a tricyclic orthoamide; experimental and theoretical evidence for C-H⋯O hydrogen bonds. Angew Chem Int Ed 26(11):1175–1177
Thakur TS, Kirchner MT, Bläser D, Boese R, Desiraju GR (2010) C-H⋯F–C hydrogen bonding in 1, 2, 3, 5-tetrafluorobenzene and other fluoroaromatic compounds and the crystal structure of alloxan revisited. CrystEngComm 12(7):2079–2085
Saha S, Rajput L, Joseph S, Mishra MK, Ganguly S, Desiraju GR (2015) IR spectroscopy as a probe for C-H⋯ X hydrogen bonded supramolecular synthons. CrystEngComm 17(6):1273–1290
Hunter CA (1994) Meldola lecture. The role of aromatic interactions in molecular recognition. Chem Soc Rev 23(2):101–109
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA , Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Peralata JE, Olgiaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghvachari K, Rendell A, Burrant JC, Iyengar SS, Tomasi J, Cossi M, Raga N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz VJ, Cioslowski, Fox DJ (2010) Gaussian Inc, Wallingford CT Gaussian 09, Revision C01
Lee C, Yang W, Parr RG (1988) Results obtained with the correlation energy density functionals. Phys Rev B Condens Matter 37:785
Francl MM, Pietro WJ, Hehre WJ, Binkley JS, Gordon MS, DeFrees DJ, Pople JA (1982) Self-consistent molecular orbital methods XXIII. A polarization-type basis set for second-row elements. J Chem Phys 77(7):3654–3665
Clark T, Chandrasekhar J, Spitznagel GW, Schleyer PVR (1983) Efficient diffuse function-augmented basis sets for anion calculations. III. The 3–21+ G basis set for first-row elements. J Comput Chem 4(3):294–301
Dickson RM, Becke AD (1993) Basis-set-free local density functional calculations of geometries of polyatomic molecules. J Chem Phys 99(5):3898–3905
Gordon MS (1980) The isomers of silacyclopropane. Chem Phys Lett 76(1):163–168
Jensen F (2007) Introduction to computational chemistry. Angew Chem Int Ed
Frisch A, Nielsen AB, Holder AJ (2001) Gauss View molecular visualization program. Gaussian Inc, Gaussian user manual
Fukui K (1981) The path of chemical reactions-the IRC approach. Acc Chem Res 14(12):363–368
Marenich AV, Cramer CJ, Truhlar DG (2009) Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J Phys Chem B 113(18):6378–6396
Bader R (1990) Atoms in molecules: a quantum theory: Oxford Univ. Press, Oxford
Zlotin G, Kislitsin G, Samet V, Serebryakov A, Konyushkin D, Semenov VV, Gakh AA (2000) Synthetic utilization of polynitroaromatic compounds. 1. S-derivatization of 1-substituted 2, 4, 6-trinitrobenzenes with thiols. J Organomet Chem 65(25):8430–8438
Rozas I, Alkorta I, Elguero J (2000) Behavior of ylides containing N, O, and C atoms as hydrogen bond acceptors. J Am Chem Soc 122(45):11154–11161
Funding
Funding is provided by Sri Guru Teg Bahadur Khalsa College, Sri Anandpur Sahib.
Author information
Authors and Affiliations
Contributions
Amanjot and Kulvinder Singh done the basic studies and helped with manuscript preparation, Amrit Singh and Pratibha Sharma performed computational evaluation of data and edited the manuscript, Amritpal Singh and Raj Kumar decided the problem for the present research work, Manish Dev Sharma provided the platform to perform Gaussian software calculations for the present work, and Amandeep Saroa performed computational evaluation of data and also wrote and edited the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in studies were in accordance with the ethical standards.
Consent to participate
Not applicable.
Consent for publication
All authors give consent to publish the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Amanjot, Kumar, R., Singh, A. et al. Comparative computational studies for nucleophilic aromatic substitution of dinitro-substituted benzannulated heterocycles with 1H-1,2,3-triazole. Struct Chem 34, 505–517 (2023). https://doi.org/10.1007/s11224-022-01993-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01993-1