Abstract
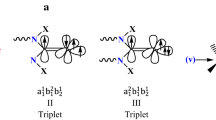
Carbenes are anticipated to interact with different greenhouse gases including CH4, N2O, CO2, etc. Here, CO2 trapping of singlet (s) and triplet (t) 4-vinylidencyclopentene minima (1s and 1t) are compared and contrasted with their various N-substituted analogs, with different types of topology (2s-9s and 2t-9t, respectively), at M06-2X/6–311 + + G** level of theory. In the first step, spontaneous non-covalent adsorption of CO2, without any transition state (TS), over singlet 1s-9s, and triplet 1t-9t, gives exothermic CO2-adducts 1s(a)-9s(a) and 1t(a)-9t(a), respectively. In the second step, the adsorbed CO2 interacts covalently with the carbene moiety of singlet 1s(a)-9s(a), and forms reactant-like, three-membered cyclic TSs that convert to their corresponding singlet 1s(b)-9s(b) exothermically. Alternatively, CO2 intramolecular reactions within triplet 1t-9t results in formation of the reactant-like TSs, which lead to the exothermic formation of triplet 1t(b)-9t(b). Singlet cyclic vinylidenes (1s-9s, with trapping energy range, Etrp = − 19.85 to − 40.34 kcal mol−1) are better CO2 trappers than their triplet spin isomers (1t-9t, with Etrp = + 1.34 to − 10.13 kcal mol−1). The best singlet and triplet trappers appeared to be Arduengo type 4s and 4t with Etrp values of − 40.34 and − 10.13 kcal mol−1, respectively. The ease of CO2 trapping by the scrutinized singlet and triplet vinylidenes is: 4s > 7s > 9s > 2s > 5s > 6s > 8s > 1s > 3s > 4t > 3t > 7t > 6t > 5t > 9t > 2t > 8t > 1t. The AIM results appear consistent with above trend and our proposed mechanism.
Graphical abstract









Similar content being viewed by others
References
Liu Q, Wu L, Jackstell R, Beller M (2015) Nat Commun 6:5933–5948
Paul HM (2016) Feron. Absorption–based post–combustion capture of carbon dioxide. Elsevier, Amsterdam, 1st ed
Suib SL (2013) New and future developments in catalysis, 1st edn. Activation of carbon dioxide. Elsevier, Amsterdam
Yang L, Wang H (2014) Chemsuschem 7:962–998
Buβ F, Mehlmann P, Mück-Lichtenfeld C, Bergarder K, Dielmann F (2016) J Am Chem Soc 138:1840–1843
Villiers C, Dognon J-P, Pollet R, Thuéry P, Ephritikhine M (2010) Angew Chem Int Ed 49:3465–3468
Kayaki Y, Yamamoto M, Ikariya T (2009) Angew Chem Int Ed 48:4194–4197
Zhou H, Zhang WZ, Liu CH, Qu JP, Lu XB (2008) J Organ Chem 73(20):8039-8044
Khorshidvand N, Kassaee MZ, Safaei S (2020) Res Chem Intermed 46:2289–2308
Montero-Campillo MM, Alkorta I, Elguero J (2018) Phys Chem Chem Phys 20:19552–19559
Ajitha MJ, Suresh CH (2012) J Org Chem 77:1087–1094
Logdi R, Bag A, Tiwari AK (2019) J Mol Graph and Mod 93:107437–107446
Vogt M, Bennett JE, Huang Y, Wu C, Schneider WF, Brennecke JF, Ashfeld BL (2013) Chem - A Eur J 19:11134–11138
Kuhn N, Steimann M, Weyers G (1999) Z Naturforsch 54:434–440
Van Ausdall BR, Glass JL, Wiggins KM, Aarif AM, Louie J (2009) J Org Chem 74:7935–7942
Alkorta I, Blanco F, Dobado A, Ferrer SM (2009) J Phys Chem A 113:8387–8393
Alkorta I, Montero-Campillo MM, Elguero J (2017) Chem - A Eur J 23:10604–10609
Denning DM, Falvey DE (2014) J Org Chem 79:4293–4299
Denning DM, Falvey DE (2017) J Org Chem 82:1552–1557
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Kosekl S, Matsunaga N, Nguyen KA, Su S, Windus TL, Duplus M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Zhao Y, Truhlar DG (2008) Theor Chem Acc 120:215–241
Valero R, Gomes JRB, Truhlar DG, lllas F (2008) J Chem Phys 129:124710–124723
Soleimani Purlak N, Kassaee MZ (2020) J Phys Org Chem 33:e4053
Ayoubi-Chianeh M, Kassaee MZ (2020) J Chinese Chem Soc 67:692–702
Seif A, Ebrahimi S, Vessally E, Goodarzi M (2013) Struct Chem 24:1737–1745
Fukui K (1981) Acc Chem Res 14:363–368
Bader RFW, Nguyen-Dang TT (1981) Adv Quantum Chem 14:63–124
Keith TA (2010) AIMAll (version 10.07.25), https://www.aim.tkgristmill.com
Siegelman RL, Milner PJ, Forse AC, Lee JH, Colwell KA, Neaton JB, Reimer JA, Weston SC, Long JR (2019) J Am Chem Soc 141:13171–13186
Li X, Guo T, Zhu L, Ling C, Xue Q, Xing W (2018) Chem Eng J 338:92–98
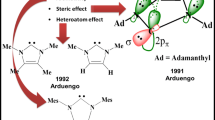
Arduengo AJ, Harlow RL, Kline M (1991) J Am Chem Soc 113:361–363
Bader RFW (1991) Chem Rev 91:893–928
Seif A, Bagherzadeh R, Goodarzi M, Azizi K (2013) J Chem Sci 125:1277–1284
Del Bene JE, Alkorta I, Elguero J (2017) J Phys Chem 121:4039–4047
Acknowledgements
The support from Tarbiat Modares University (TMU) is gratefully acknowledged.
Funding
This study is financially supported by Tarbiat Modares University.
Author information
Authors and Affiliations
Contributions
Shadi Soroudi. Mohamad Zaman Kassaee.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soroudi, S., Kassaee, M.Z. Effects of N-substitution on CO2 trapping by cyclic vinylidenes at DFT levels. Struct Chem 34, 467–476 (2023). https://doi.org/10.1007/s11224-022-01977-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01977-1