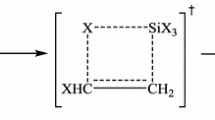
Abstract
This paper features the results of the computational study of thermal decomposition reaction of 5-nitro-5-R-1,3-dioxane compounds, with R = H, Br, and CH3. Computational calculations were performed with M06-2X, MPWB1K, PBE0 and ωB97X-D functionals, and 6–311 + G(d,p) basis set in gas phase and also in solution with DMSO, at different temperatures. The kinetic and thermodynamic data obtained indicate a favoring of the reaction when the molecule presents substituent groups in position 5 and when carried out in DMSO, the stability of the molecules in their energetic components was discussed, too. For R = H two different reaction mechanisms were proposed and studied. Wiberg bond indices were obtained for the reactions studied and the results were examined in terms of bond formation and bond breaking progress as well.






Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Carrera G, Vegué L, Boleda MR, Ventura F (2017) Simultaneous determination of the potential carcinogen 1,4-dioxane and malodorous alkyl-1,3-dioxanes and alkyl-1,3-dioxolanes in environmental waters by solid-phase extraction and gas chromatography tandem mass spectrometry. J Chromatogr A 1487:1–13
Kuramshina AE, Kuznetsov VV (2010) Quantum-chemical study of 1,3-dioxane complexes with two water molecules. Russ J Org Chem 46:665–669
Zeng L, Xu G, Gao P, Zhang M, Li H, Zhang J (2015) Design, synthesis and evaluation of a novel class of glucosamine mimetic peptides containing 1,3-dioxane. Eur J Med Chem 93:109–120
Wong JC, Sternson SM, Louca JB, Hong R, Schreiber SL (2004) Modular synthesis and preliminary biological evaluation of stereochemically diverse 1,3-dioxanes. Chem Biol 11:1279–1291
Lapass LC, Hirsch CA, Winely CL (1976) Substituted 5-nitro-1,3 -dioxanes: correlation of chemical structure and antimicrobial activity. J Pharm Sci 65:1301–1305
Adelaide T, Adebayo W, Rusell B, Salt WG (1987) Radical-nucleophilic substitution (SRN1) reactions. Part 5. Anions of nitroimidazoles in SRN1 and oxidative addition reactions. J Chem Soc Perkin Trans I:2819–2827
Fernandez-Alvarez M, Lamas JP, Sanchez-Prado L, Llompart M, Garcia-Jares C, Lores M (2010) Development of a solid-phase microextraction gas chromatography with microelectron-capture detection method for the determination of 5-bromo-5-nitro-1,3-dioxane in rinse-off cosmetics. J Chromatogr A 1217:6634–6639
Stepanov RS, Kruglyakova LA, Astakhov AM, Golubtsova OA (2004) Kinetics and mechanism of thermal decomposition of 2-substituted 5,5-dinitro-1,3-dioxanes. Russ J Gen Chem 74:1579–1582
Lytko-Krasuska A, Piotrowska H, Urbanski T (1979) Reductive elimination of a tertiary nitro group in 5-nitro- 1,3-dioxanes. Tetrahedron Lett 20:1243–1246
Stepanov RS, Kruglyakova LA, Golubtsova OA (2011) Kinetics of thermolysis of 5-nitro-5-R-1,3-dioxanes. Russ J Gen Chem 81:151–152
Kupova OY, Vakulin IV, Talipov RF (2013) Ab initio study of 1,3-dioxanes formation from formaldehyde dimer and alkenes. Comput Theor Chem 1013:57–61
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, Revision B.01, Gaussian Inc., Wallingford CT
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Acc 120:215–241
Ditchfield R, Hehre WJ, Pople JA (1971) Self-consistent molecular-orbital methods. IX. An extended Gaussian-type basis for molecular-orbital studies of organic molecules. J Chem Phys 54:724–728
Zhao Y, Truhlar DG (2004) Hybrid meta density functional theory methods for thermochemistry, thermochemical kinetics, and noncovalent interactions: the MPW1B95 and MPWB1K models and comparative assessments for hydrogen bonding and van der Waals interactions. J Phys Chem A 108:6908–6918
Adamo C, Barone V (1999) Toward reliable density functional methods without adjustable parameters: The PBE0 model. J Chem Phys 110:6158–6170
Chai JD, Head-Gordon M (2008) Long-range corrected hybrid density functionals with damped atom–atom dispersion corrections. Phys Chem Chem Phys 10:6615–6620
Merrick JP, Moran D, Radom L (2007) An evaluation of harmonic vibrational frequency scale factors. J Phys Chem A 111:11683–11700
McQuarrie DA, Simon JD (1999) Molecular thermodynamics. University Science Books, Sausalito, CA
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094
Fukui K (1970) A formulation of the reaction coordinate. J Phys Chem 74:4161–4163
Glasstone S, Laidler K, Eyring H (1941) In: The theory of rate processes, 1st edn. McGraw Hill, New York
Benson SW (1969) The Foundations of chemical kinetics. McGraw-Hill, New York
Reed AE, Weinhold FJ (1983) Natural bond orbital analysis of near-Hartree–Fock water dimer. Chem Phys 78:4066–4073
Reed AE, Curtiss LA, Weinhold F (1988) Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem Rev 88:899–926
Glendening ED, Reed AE, Carpenter JE, Weinhold F (1988) NBO Version 3.1. Madison WI
Wiberg KB (1968) Application of the pople-santry-segal CNDO method to the cyclopropylcarbinyl and cyclobutyl cation and to bicyclobutane. Tetrahedron 24:1083–1096
Moyano A, Pericas MA, Valentí E (1989) A theoretical study on the mechanism of the thermal and the acid-catalyzed decarboxylation of 2-oxetanones (β-lactones). J Org Chem 54:573–582
Acknowledgements
The authors are grateful for the support received from the Universidad Nacional de Colombia – Medellin Headquarters during the research and publication stages of this work. S. Q. also thanks Dirección General de Investigaciones of Universidad Santiago de Cali (DGI), Santiago de Cali. P. R. also thanks Instituto Tecnológico Metropolitano (ITM).
Author information
Authors and Affiliations
Contributions
The contribution of each author listed is based on Silvia Quijano and Pablo Ruiz, who made a substantial contribution to the conception and design, data acquisition and analysis, and interpretation of the data; Pablo Ruiz and Jairo Quijano drafted the article and critically reviewed the intellectual content. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ruiz, P., Quijano, S. & Quijano, J. Computational investigation of thermal decomposition mechanism of 5-nitro-5-R-1,3-dioxane compounds. Struct Chem 33, 1149–1155 (2022). https://doi.org/10.1007/s11224-022-01891-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-022-01891-6