Abstract
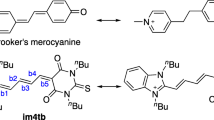
The concept of the ideal polymethine state lies at the heart of the theory of color of π-conjugated organic compounds. For merocyanines—neutral donor–acceptor polymethine dyes—an attainment of a structure close to the cyanine limit is usually traced in solutions by their solvatochromic behavior or 1H NMR spectra. Herein, we report an X-ray crystallographic study of a vinylogous series of merocyanines characterized by a close uniformity of the C − C-bond length in the polymethine chain in the solid state. It reveals that for merocyanines in the crystal the ideal polymethine state can be achieved via a judicious choice of the donor–acceptor properties of their end-groups. The polymethine chain length is found to have only a slight impact on the electronic symmetry of such dyes in the solid state, as distinct from both less and more dipolar merocyanines, the bond length alternation in the chromophore of which increases for higher vinylogues. The interaction energies between merocyanine molecules in the stacking dimers in the crystal have been analyzed using the Localized Molecular Orbital Energy Decomposition Analysis (LMOEDA) algorithm.







Similar content being viewed by others
Availability of data and materials
Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre, 11 Union Road, Cambridge, CB2 1EZ, UK (fax,: + 44 1223 336,033; e-mail, deposit@ccdc.cam.ac.uk): deposition numbers CCDC 2,082,392–2,082,396. All other data supporting the findings of this study are available within the article and its supplementary material.
References
Dähne S (1978) Color and constitution: one hundred years of research. Science 199:1163–1167. https://doi.org/10.1126/science.199.4334.1163
Dähne S (1991) Der ideale Polymethinzustand. Chimia 45:288–296
Bach G, Dähne S (1997) Cyanine dyes and related compounds. In: Sainsbury M (ed) Second Supplements to the 2nd Edition of Rodd’s Chemistry of Carbon Compounds: A Modern Comprehensive Treatise. Elsevier, Amsterdam, pp 383–481
Marder SR, Perry JW, Tiemann BG et al (1993) Direct observation of reduced bond length alternation in donor/acceptor polyenes. J Am Chem Soc 115:2524–2526. https://doi.org/10.1021/ja00059a067
Murugan NA, Aidas K, Kongsted J et al (2012) NMR spin-spin coupling constants in polymethine dyes as polarity indicators. Chem Eur J 18:11677–11684. https://doi.org/10.1002/chem.201200270
Le Guennic B, Jacquemin D (2015) Taking up the cyanine challenge with quantum tools. Acc Chem Res 48:530–537. https://doi.org/10.1021/ar500447q
Pascal S, Getmanenko YA, Zhang Y et al (2018) Design of near-infrared-absorbing unsymmetrical polymethine dyes with large quadratic hyperpolarizabilities. Chem Mater 30:3410–3418. https://doi.org/10.1021/acs.chemmater.8b00960
Eskandari M, Roldao JC, Cerezo J et al (2020) Counterion-mediated crossing of the cyanine limit in crystals and fluid solution: bond length alternation and spectral broadening unveiled by quantum chemistry. J Am Chem Soc 142:2835–2843. https://doi.org/10.1021/jacs.9b10686
Capobianco A, Borrelli R, Landi A et al (2016) Absorption band shapes of a push-pull dye approaching the cyanine limit: a challenging case for first principle calculations. J Phys Chem A 120:5581–5589. https://doi.org/10.1021/acs.jpca.6b05220
Kulinich AV, Ishchenko AA (2009) Merocyanine dyes: synthesis, structure, properties and applications. Russ Chem Rev 78:141–164. https://doi.org/10.1070/rc2009v078n02abeh003900
Pascal S, Haefele A, Monnereau C et al (2014) Expanding the polymethine paradigm: evidence for the contribution of a bis-dipolar electronic structure. J Phys Chem A 118:4038–4047. https://doi.org/10.1021/jp501358q
Haenle JC, Bruchlos K, Ludwigs S et al (2017) Rigidified Push-Pull Dyes: Using chromophore size, donor, and acceptor units to tune the ground state between neutral and the cyanine limit. Chem Plus Chem 82:1197–1210. https://doi.org/10.1002/cplu.201700347
Ishchenko AA, Derevyanko NA, Zubarovskii VM, Tolmachev AI (1984) Influence of length of the polymethine chain on width of absorption bands of symmetric cyanine byes. Theor Exp Chem 20:415–422. https://doi.org/10.1007/BF00516576
Tolbert LM, Zhao X (1997) Beyond the cyanine limit: Peierls distortion and symmetry collapse in a polymethine dye. J Am Chem Soc 119:3253–3258. https://doi.org/10.1021/ja9626953
Fabian J (2006) Symmetry-lowering distortion of near-infrared polymethine dyes-a study by first-principles methods. J Mol Struct THEOCHEM 766:49–60. https://doi.org/10.1016/j.theochem.2006.02.003
Masunov AE, Anderson D, Freidzon AY, Bagaturyants AA (2015) Symmetry-breaking in cationic polymethine dyes: part 2. Shape of electronic absorption bands explained by the thermal fluctuations of the solvent reaction field. J Phys Chem A 119:6807–6815. https://doi.org/10.1021/acs.jpca.5b03877
Brooker LGS, Keyes GH, Sprague RH et al (1951) Color and constitution. X.1 Absorption of the merocyanines2. J Am Chem Soc 73:5332–5350. https://doi.org/10.1021/ja01155a096
Murugan NA, Kongsted J, Rinkevicius Z, Ågren H (2011) Demystifying the solvatochromic reversal in Brooker’s merocyanine dye. Phys Chem Chem Phys 13:1290–1292. https://doi.org/10.1039/c0cp01014f
Hoche J, Schulz A, Dietrich LM et al (2019) The origin of the solvent dependence of fluorescence quantum yields in dipolar merocyanine dyes. Chem Sci 10:11013–11022. https://doi.org/10.1039/c9sc05012d
Seifert S, Seifert A, Brunklaus G et al (2012) Probing the surface polarity of inorganic oxides using merocyanine-type dyes derived from barbituric acid. New J Chem 36:674–684. https://doi.org/10.1039/c2nj20835k
Ashoka AH, Ashokkumar P, Kovtun YP, Klymchenko AS (2019) Solvatochromic near-infrared probe for polarity mapping of biomembranes and lipid droplets in cells under stress. J Phys Chem Lett 10:2414–2421. https://doi.org/10.1021/acs.jpclett.9b00668
Chen KW, Huang CW, Lin SY et al (2015) Merocyanines for vacuum-deposited small-molecule organic solar cells. Org Electron 26:319–326. https://doi.org/10.1016/j.orgel.2015.07.050
Arjona-Esteban A, Lenze MR, Meerholz K, Würthner F (2017) Donor–acceptor dyes for organic photovoltaics. In: Leo K (ed) Elementary processes in organic photovoltaics. Adv Polym Sci. Springer, Cham, pp 193–214
Bulliard X, Jin YW, Lee GH et al (2016) Dipolar donor-acceptor molecules in the cyanine limit for high efficiency green-light-selective organic photodiodes. J Mater Chem C 4:1117–1125. https://doi.org/10.1039/c5tc03567h
Hales JM, Barlow S, Kim H et al (2014) Design of organic chromophores for all-optical signal processing applications. Chem Mater 26:549–560. https://doi.org/10.1021/cm402893s
Castet F, Blanchard-Desce M, Adamietz F et al (2014) Experimental and theoretical investigation of the first-order hyperpolarizability of octupolar merocyanine dyes. ChemPhysChem 15:2575–2581. https://doi.org/10.1002/cphc.201402083
Brandão I, Franco LR, Fonseca TL et al (2017) Confirming the relationship between first hyperpolarizability and the bond length alternation coordinate for merocyanine dyes. J Chem Phys 146:224505. https://doi.org/10.1063/1.4985672
Viteva L, Gospodova T, Rashkova J et al (2007) Synthesis and photophysical properties of some rigidized hepta- and nonamethine mono- and bis(merocyanines): ring-opening of quaternized 2-methylbenzothiazole. European J Org Chem 3102–3114. https://doi.org/10.1002/ejoc.200700125
Yagai S, Kinoshita T, Higashi M et al (2007) Diversification of self-organized architectures in supramolecular dye assemblies. J Am Chem Soc 129:13277–13287. https://doi.org/10.1021/ja075257c
Bialas D, Zitzler-Kunkel A, Kirchner E et al (2016) Structural and quantum chemical analysis of exciton coupling in homo-and heteroaggregate stacks of merocyanines. Nat Commun 7:12949. https://doi.org/10.1038/ncomms12949
Liess A, Huang L, Arjona-Esteban A et al (2015) Organic thin film transistors based on highly dipolar donor-acceptor polymethine dyes. Adv Funct Mater 25:44–57. https://doi.org/10.1002/adfm.201402678
Chan YH, Gallina ME, Zhang X et al (2012) Reversible photoswitching of spiropyran-conjugated semiconducting polymer dots. Anal Chem 84:9431–9438. https://doi.org/10.1021/ac302245t
Kreß KC, Fischer T, Stumpe J et al (2014) Influence of chromophore length and acceptor groups on the optical properties of rigidified merocyanine dyes. Chem Plus Chem 79:223–232. https://doi.org/10.1002/cplu.201300308
Ishchenko AA, Kulinich AV, Shishkina SV (2017) Effect of donor terminal group and polymethine chain length on structure of merocyanine dyes in the crystal state. Dyes Pigm 145:181–188. https://doi.org/10.1016/j.dyepig.2017.06.009
Tillotson JP, Bogdanov G, Jucov EV et al (2019) Synthesis, structure, linear and nonlinear properties of tricyanofuran–terminated merocyanine dyes. J Mol Struct 1189:146–154. https://doi.org/10.1016/j.molstruc.2019.04.001
Rigin S, Tillotson J, Perry J et al (2020) Polymorphism of merocyanine dyes homologues with 1,3-diethyl-2-thiobarbituric acid acceptor and p-dimethylaminobenzene donor and different polymethine chains connecting Them. Cryst Growth Des 20:167–177. https://doi.org/10.1021/acs.cgd.9b00961
Pauk K, Luňák S, Růžička A et al (2021) Green-, red-, and infrared-emitting polymorphs of sterically hindered push–pull substituted stilbenes. Chem Eur J 27:4341–4348. https://doi.org/10.1002/chem.202004419
Ishchenko AA, Kulinich AV, Bondarev SL, Knyukshto VN (2007) Photodynamics of polyene-polymethine transformations and spectral fluorescent properties of merocyanine dyes. J Phys Chem A 111:13629–13637. https://doi.org/10.1021/jp076016u
Ishchenko AA, Kulinich AV, Bondarev SL, Knyukshto VN (2008) Electronic structure and fluorescent properties of malononitrile-based merocyanines with positive and negative solvatochromism. Opt Spectrosc 104:57–68. https://doi.org/10.1134/s0030400x08010086
Würthner F, Wortmann R, Matschiner R et al (1997) Merocyanine dyes in the cyanine limit: a new class of chromophores for photorefractive materials. Angew Chem Int Ed 36:2765–2768. https://doi.org/10.1002/anie.199727651
Mustroph H, Reiner K, Senns B et al (2012) The effects of substituents and solvents on the ground-state π-electronic structure and electronic absorption spectra of a series of model merocyanine dyes and their theoretical interpretation. Chem Eur J 18:8140–8149. https://doi.org/10.1002/chem.201101830
Rezende MC (2016) A generalized reversal model for the solvatochromism of merocyanines. J Phys Org Chem 29:460–467. https://doi.org/10.1002/poc.3565
Sturmer DM, Heseltine DW (1977) Sensitizing and desensitizing dyes. In: James TH (ed) The theory of the photographic process, 4th edn. Macmillan Pub Co, New York, pp 194–234
Kulinich AV, Derevyanko NA, Ishchenko AA (2005) Synthesis and spectral properties of malononitrile-based merocyanine dyes. Russ Chem Bull 54:2820–2830. https://doi.org/10.1007/s11172-006-0196-0
Strell M, Braunbruck WB, Fühler WF, Huber O (1954) Polymethin-Farbstoffe I. Über Cyanomethine Liebigs Ann Chem 587:177–194. https://doi.org/10.1002/jlac.19545870302
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr E64:112–122. https://doi.org/10.1107/S0108767307043930
Frisch MJ, Trucks GW, Schlegel HB et al (2009) Gaussian 09, Rev. D.01. Gaussian Inc., Wallingford, CT
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652. https://doi.org/10.1063/1.464913
Pople JA, Schleyer PV, Hehre WJ, Radom L (1986) AB INITIO molecular orbital theory. Wiley, New York
Tomasi J, Mennucci B, Cammi R (2005) Quantum mechanical continuum solvation models. Chem Rev 105:2999–3094. https://doi.org/10.1021/cr9904009
Kitaura K, Morokuma K (1976) A new energy decomposition scheme for molecular interactions within the Hartree-Fock approximation. Int J Quantum Chem 10:325–340. https://doi.org/10.1002/qua.560100211
Su P, Li H (2009) Energy decomposition analysis of covalent bonds and intermolecular interactions. J Chem Phys 131:014102. https://doi.org/10.1063/1.3159673
Barca GMJ, Bertoni C, Carrington L et al (2020) Recent developments in the general atomic and molecular electronic structure system. J Chem Phys 152:154102. https://doi.org/10.1063/5.0005188
Zhao Y, Truhlar DG (2008) The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other function. Theor Chem Acc 120:215–241. https://doi.org/10.1007/s00214-007-0310-x
Boys SF, Bernardi F (1970) The calculation of small molecular interactions by the differences of separate total energies. Some procedures with reduced errors. Mol Phys 19:553–566. https://doi.org/10.1080/00268977000101561
Liu Q, Wang X, Wang LY et al (2011) The synthesis, spectroscopic properties and crystal structures of some rhodanine merocyanine dyes for optical recording with a blue diode laser. Dyes Pigm 91:370–377. https://doi.org/10.1016/j.dyepig.2011.03.030
Hayes KL, Lasher EM, Choczynski JM et al (2018) Brooker’s merocyanine: comparison of single crystal structures. J Mol Struct 1161:194–198. https://doi.org/10.1016/j.molstruc.2018.02.050
Seiler VK, Callebaut K, Robeyns K et al (2018) Acidochromic spiropyran-merocyanine stabilisation in the solid state. CrystEngComm 20:3318–3327. https://doi.org/10.1039/c8ce00291f
Shishkina SV, Ishchenko AA, Kulinich AV (2021) Structure and intermolecular interactions of the fully negative solvatochromic merocyanine in the crystal phase. Struct Chem 32:1341–1345. https://doi.org/10.1007/s11224-021-01774-2
Ishchenko AA (1991) Structure and spectral-luminescent properties of polymethine dyes. Russ Chem Rev 60:865–884. https://doi.org/10.1070/rc1991v060n08abeh001116
Kulinich AV, Ishchenko AA, Bondarev SL, Knyukshto VN (2018) Low-temperature effect on the electronic structure and spectral-fluorescent properties of highly dipolar merocyanines. J Phys Chem A 122:9645–9652. https://doi.org/10.1021/acs.jpca.8b09522
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection and analysis were performed by Svitlana V. Shishkina, Viktoriya V. Dyakonenko, and Andrii V. Kulinich. The first draft of the manuscript was written by Andrii V. Kulinich, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shishkina, S.V., Dyakonenko, V.V., Ishchenko, A.A. et al. Ideal polymethine state of merocyanines in the crystal. Struct Chem 33, 169–178 (2022). https://doi.org/10.1007/s11224-021-01834-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01834-7