Abstract
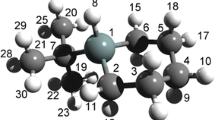
The equilibrium structure of the 3,3,6-trimethyl-1,5-diazabicyclo[3.1.0]hexane (TMDABH) molecule was studied for the first time by means of gas-phase electron diffraction (GED) supplemented with quantum chemical calculations. TMDABH exists as a mixture of two conformers of Cs point group symmetry, namely, a chair and a boat. The fractions of these two forms are 74 and 26% respectively, if the reference approximation was obtained at the MP2/aug-cc-PVTZ level, and 81 and 19%, if the reference approximation was generated at the DFT-B3LYP/cc-pVTZ level. The agreement between the theoretical and experimental molecular intensities is characterized by Rf disagreement factors of 5.06 and 6.93%, respectively. Based on the more accurate MP2/aug-cc-PVTZ quantum chemical approximation, the energy difference between the global minimum, which corresponds to the chair conformer of TMDABH, and the local minimum of the boat on the potential energy surface (PES) was found to be 1.39 kcal/mol. Moreover, NMR, IR, and Raman spectroscopic studies were carried out. According to the joint analysis of the data obtained, the most important equilibrium parameters of the chair and boat TMDABH conformers were determined to be as follows (bond lengths in Å, angles in degrees, for boat form in square brackets, Cs symmetry): N1N5 = 1.554(2) [1.520(2)], C2C3 = 1.525(2) [1.539(2)], N1C2C3 = 105.7(14) [108.8(14)], θ = C2-C3-C4/C2-N1-N5-C4 = 37.4(6) [−16.6(6)], φ = N1-C6-N5/C2-N1-N5-C4 = 72.9(3) [73.9(3)]. Comparison of especially NN bond lengths reveals a strong dependence on the bicyclic system conformation.







Similar content being viewed by others
Availability of data and material
All data generated or analyzed during this study are included in this published paper [and its supplementary information files].
References
Gessner KJ, Ball DW (2005) J Mol Struct: THEOCHEM 730:95–103
Vishnevskiy YV, Schwabedissen J, Rykov AN, Kuznetsov VV, Makhova NN (2015) J Phys Chem A 119:10871–10881
Shustov GV, Kadorkina GK, Varlamov SV, Kachanov AV, Kostyanovskii RG, Rauk A (1992) J Am Chem Soc 114:1616–1623
Kamuf M, Trapp O (2011) Chirality 23:113–117
Paget CJ, Davis CS (1964) J Med Chem 7:626–628
Baichurina AZ, Semina VI, Garaev RS (1996) Bull Exp Biol Med 121:584–586
Makhova NN, Petukhova VY, Shevtsov AV, Novakovskiy VV, Kuznetsov VV (2013) Agents for treating neurodegenerative disorders, US Pat., WO2013/111117A2
Makhova NN, Petukhova VY, Shevtsov AV, Novakovskiy VV, Kuznetsov VV (2013) Agents for treating neurodegenerative disorders, US Pat., WO2013/111118A2
Makhova NN, Petukhova VY, Shevtsov AV, Novakovskiy VV, Kuznetsov VV (2013) Agents for treating neurodegenerative disorders, US Pat., WO2013.121334A2
Syroeshkina YS, Kuznetsov VV, Lyssenko KA, Makhova NN (2009) Russ Chem Bull 58:366–379
Kuznetsov VV, Kutepov SA, Makhova NN, Lyssenko KA, Dmitriev DE (2003) Russ Chem Bull Int Ed 52:665–673
Makhova NN, Petukhova VY, Kuznetsov VV (2008) ARKIVOC, i, 128
Makhova NN, Shevtsov AV, Petukhova VY (2011) Russ Chem Rev 80:1085–1133
Vishnevskiy YV, Vogt N, Vogt J, Rykov AN, Kuznetsov VV, Makhova NN, Vilkov LV (2008) J Phys Chem A 112:5243–5250
Atavin EG, Golubinsky AV, Popik MV, Kuznetsov VV, Makhova NN, Vilkov LV (2003) J Struct Chem 44:779–783
Khaikin LS, Ageev GG, Rykov AN, Grikina OE, Shishkov IF, Kochikov IV, Kuznetsov VV, Makhova NN, Bukalov SS, Leites LA (2020) Phys Chem Chem Phys 22:22477–22492
Shevtsov AV, Kuznetsov VV, Kislukhin AA, Petukhova VYu, Strelenko YuA, Makhova NN (2006) J Heterocycl Chem 43:881–888
Chagarovskiy AO, Vasin VS, Kuznetsov VV, Ivanova OA, Rybakov VB, Shumsky AN, Makhova NN, Trushkov IV (2018) Angew Chem Int Ed 57:10338–10342
Chagarovskiy AO, Kuznetsov VV, Ivanova OA, Goloveshkin AS, Levina II, Makhova NN, Trushkov IV (2019) Eur J Org Chem 2019:5475–5485
Pitzer K, Donath WE (1959) J Am Chem Soc 81:3213–3218
Romers C, Altona C, Buys HR, Havinga E (1969) Top Stereochem 4:39–97
Fuchs B (1978) Top Stereochem 10:1–94
Greenberg A, Liebman JF (1978) Strained Organic Molecules. Academic Press, New York
Zhang X, Senay L et al (2017) In Eng Chem Res 56:2883–2888
Kuznetsov VV, Kachala VV, Makhova NN (2018) Mendeleev Commun 28:497–500
Kochikov IV, Kovtun DM, Tarasov YI (2008) A new software for processing the radial symmetric diffractograms, in Numerical methods and programming. Section 2, Programming (Vychislitel’nye Metody i Programmirovanie) 9:12–18
Kochikov IV, Tarasov YuI, Ivanov AA (2007) J Struct Chem 48:558–563
Ross AW, Fink M, Hilderbrandt RL (1999) In: Wilson AJC (ed) International tables for X-ray crystallography, Vol. 4. Kluwer, Dordrecht
Tavard C, Nicolas D, Rouault M (1967) J Chem Phys 64:540–554
Spiridonov VP (1997) in Advances in molecular structure research, ed. Hargittai I and Hargittai M, JAI. Greenwich 3:53–81
Spiridonov VP, Vogt N, Vogt J (2001) Struct Chem 12:349–376
Ischenko AA, Girichev GV, Tarasov YI (2013) Electron diffraction: Structure and dynamics of free molecules and condensed state of substance. Fizmatlit, Moscow (in Russian)
Ischenko AA (2018) Structure and dynamics of free molecules and condensed matter. Fizmatlit, Moscow (in Russian)
Kochikov IV, Tarasov YI, Kuramshina GM, Spiridonov VP, Yagola AG, Strand TG (1998) J Mol Struct 445:243–258
Kochikov IV, Tarasov YI, Spiridonov VP, Kuramshina GM, Yagola AG, Saakjan AS, Popik MV, Samdal S (1999) J Mol Struct 485–486:421–443
Kochikov IV, Tarasov YI, Vogt N, Spiridonov VP (2002) J Mol Struct 607:163–174
Dakkouri M, Kochikov IV, Tarasov YI, Vogt N, Vogt J, Bitschenauer R (2002) J Mol Struct 607:195–206
Kochikov IV, Tarasov YI (2003) Struct Chem 14:227–238
Kovtun DM, Kochikov IV, Tarasov YI (2015) J Phys Chem A 119:1657–1665
Khaikin LS, Kochikov IV, Grikina OE, Tikhonov DS, Baskir EG (2015) Struct Chem 26:1651–1687
Khaikin LS, Vogt N, Rykov AN, Grikina OE, Vogt J, Kochikov IV, Ageeva ES, Shishkov IF (2018) Mendeleev Commun 28:236–238
Khaikin LS, Vogt N, Rykov AN, Grikina OE, Demaison J, Vogt J, Kochikov IV, Shishova YD, Ageeva ES, Shishkov IF (2018) Russ J Phys Chem A 92:1970–1974
Yagola AG, Kochikov IV, Kuramshina GM, Pentin YA (1999) Inverse problems of vibrational spectroscopy. VSP BV, Utrecht
Fogarasi G, Pulay P (1985) In: Durig JR (ed) Vibrational spectra and structure, Vol. 14, Chap. 3. Elsevier, Amsterdam, pp 125–219
Pulay P, Fogarasi G, Pang F, Boggs JE (1979) J Am Chem Soc 101:2550–2560
Pulay P, Fogarasi G, Pongor G, Boggs JE, Vargha A (1983) J Am Chem Soc 105:7037–7047
Hamilton W (1965) Acta Cryst 18:502–510
Kuznetsov VV, Marochkin II, Goloveshkin AS, Makhova NN, Shishkov IF (2017) Struct Chem 28:1211–1221
Acknowledgements
We respectfully dedicate this paper to the memory of our esteemed late colleague, Dr. Leonid S. Khaikin of Lomonosov Moscow State University, a research leader in the field of GED and its combined application with spectroscopy and quantum chemical calculations for the structural analysis. His long-term study of nitrogen-containing organic molecular structures together with his wife Olga E. Grikina strongly contributed to the development of an extended molecular structure database. His will to overcome obstacles inspired his colleagues to model oneself on him and hand on the torch of structural chemistry in the future.
Funding
This work was supported by the Russian Foundation for Basic Research (Projects Nos. 20–03-00747 and 19–33-90274).
Author information
Authors and Affiliations
Contributions
The corresponding author carried out a full description of quantum chemical calculations, structural analysis, provided the discussion of the results, and formulated a conclusion. A.N. Rykov carried out a gas-phase electron diffraction experiment. I.F. Shishkov was directly involved in the description and discussion. The introduction and the section devoted to the analysis of NMR spectra were written by V.V. Kuznetsov and N.N. Makhova, who also synthesized 3,3,6-trimethyl-1,5-diazabicyclo[3.1.0]hexane. Quantum chemical calculations using the Gaussian package and structural analysis with the use of Symm/Disp/Large/Eldiff software package were carried out by O.E. Grikina and I.V Kochikov. S.S. Bukalov was responsible for the NMR, IR, and Raman spectroscopic measurements.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Nina N. Makhova passed away during submission of this manuscript.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ageev, G.G., Rykov, A.N., Grikina, O.E. et al. Equilibrium Molecular Structure of 3,3,6-trimethyl-1,5-diazabicyclo[3.1.0]hexane: the joint analysis of the gas-phase electron diffraction data and quantum chemical simulations. Struct Chem 33, 113–122 (2022). https://doi.org/10.1007/s11224-021-01828-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-021-01828-5