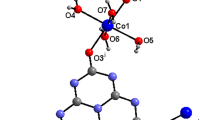
Abstract
The dynamic combinatorial library (DCL) of cyamelurates consists of 17 tautomers of cyameluric acid and their ions, as well as metal aqua complexes. That leads to a variety of compounds in the Zn(NO3)2 – K3(C6N7O3) – H2O system. All compounds are obtained with impurity amorphous and crystalline phases, which complicates their study. In this work, metastable phases of zinc, nickel, and iron cyamelurates were obtained and their crystal structures were determined for the first time. Kinetic control of chemical reactions allowed us to isolate two zinc cyamelurate metastable phases. The metastable compound K2Zn(C6N7O3H)2∙8H2O is formed immediately after mixing the precursor solutions, and after two hours, it is converted to another metastable compound Zn(C6N7O3H)∙5H2O. Due to hydrate isomerization, after 12 hours in the mother liquor, the latter transforms into a thermodynamically stable compound of the same composition. The features of the synthesis and crystalline structure of zinc cyamelurates can be interpreted from the standpoint of the nonclassical nucleation theory. The crystal structures of the synthesized compounds K2Zn(C6N7O3H)2∙8H2O, Zn(C6N7O3H)∙5H2O, and Zn(C6N7O3H2)2∙8H2O allowed us to conclude that decomposition of supersaturated zinc solution leads to the formation of double electric micelles (DEM). They can be considered nanoreactors playing an important role in crystal nucleation.

Graphical abstract









Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available in Cambridge Crystallographic Data Centre, CCDC numbers 2010959, 2010962, and 2011516 [https://www.ccdc.cam.ac.uk/].
References
Mohan M, Rajak S, Tremblay AA, Maris T, Duong A (2019) Syntheses of mono and bimetallic cyamelurate polymers with reversible chromic behaviour. Dalton Trans. https://doi.org/10.1039/C9DT01278H
Holst JR (2009) Synthesis of inorganic heptazine-based materials. PhD thesis, University of Iowa, http://ir.uiowa.edu/etd/242
Wagler J, El-Gamel NEA, Kroke E (2006) The structure and tautomerism of cyameluric acid. Z Naturforsch. https://doi.org/10.1515/znb-2006-0808
Liang X, Zheng W, Wong NB, Shu Y, Tian A (2005) Solvent-assisted catalysis mechanism on keto-enoltautomerism of cyameluric acid. THEOCHEM J Mol Struct. https://doi.org/10.1016/j.theochem.2005.05.009
Alkorta I, Jagerovic N, Elguero J (2004) Theoretical study of cyameluric acid and related compounds. ARKIVOC. https://doi.org/10.3998/ark.5550190.0005.415
Jehannin M, Rao A, Cölfen H (2019) New horizons of nonclassical crystallization. J Am Chem Soc. https://doi.org/10.1021/jacs.9b01883
Sturm EV, Cölfen H (2017) Mesocrystals: past presence future. Crystals. https://doi.org/10.3390/cryst7070207
Cölfen H, Antonietti M (2008) Mesocrystals and nonclassical crystallization. John Wiley&Sons, Chichester
Vekilov PG (2010) The two-step mechanism of nucleation of crystals in solution. Nanoscale. https://doi.org/10.1039/C0NR00628A
Gebauer D, Kellermeier M, Gale JD, Bergstromand L, Colfen H (2014) Pre-nucleation clusters as solute precursors in crystallization. Chem Soc Rev. https://doi.org/10.1039/C3CS60451A
Gebauer D, Wolf SE (2019) Designing solid materials from their solute state: a shift in paradigms toward a holistic approach in functional materials chemistry. J Am Chem Soc. https://doi.org/10.1021/jacs.8b13231
Barlow DA, Gregus J (2020) Size evolution and composition of the intermediate phase during nonclassical protein crystal growth from solution. Cryst Growth Des. https://doi.org/10.1021/acs.cgd.0c00019
Yamazaki T, Kimura Y, Vekilov PG, Furukawa E, Shirai M, Matsumoto H, Van Driessche AES, Tsukamoto K (2017) Two types of amorphous protein particles facilitate crystal nucleation. PNAS. https://doi.org/10.1073/pnas.1606948114
Sauter A, Roosen-Runge F, Zhang F, Lotze G, Jacobs RMJ, Schreiber F (2015) Real-time observation of nonclassical protein crystallization kinetics. J Am Chem Soc. https://doi.org/10.1021/ja510533x
Barlow DA, Gregus J (2019) The kinetics of homogeneous and two-step nucleation during protein crystal growth from solution. Int J Chem Kinet. https://doi.org/10.1002/kin.21313
Ostwald W (1897) Studien über die Bildung und Umwandlung fester Körper. Z Phys Chem. https://doi.org/10.1515/zpch-1897-2233
Ohtsu H, Kawano M (2017) Kinetic assembly of coordination networks. Chem Commun. https://doi.org/10.1039/C7CC04277A
Kawano M, Haneda T, Hashizume D, Izumi F, Fujita M (2008) A selective instant synthesis of a coordination network and its ab initio powder structure determination. Angew Chem Int Ed. https://doi.org/10.1002/anie.200704809
Martí-Rujas J, Islam N, Hashizume D, Izumi F, Fujita M, Kawano M (2011) Dramatic structural rearrangements in porous coordination networks. J Am Chem Soc. https://doi.org/10.1021/ja109160a
Bennett TD, Goodwin AL, Dove MT, Keen DA, Tucker MG, Barney ER, Soper AK, Bithell EG, Tan JC, Cheetham AK (2010) Structure and properties of an amorphous metal-organic framework. Phys Rev Lett. https://doi.org/10.1103/PhysRevLett.104.115503
Cheetham AK, Kieslich G, Yeung HHM (2018) Thermodynamic and kinetic effects in the crystallization of metal–organic frameworks. Acc Chem Res. https://doi.org/10.1021/acs.accounts.7b00497
Forster PM, Burbank AR, Livage C, Férey G, Cheetham AK (2004) The role of temperature in the synthesis of hybrid inorganic–organic materials: the example of cobalt succinates. Chem Commun. https://doi.org/10.1039/b311156c
Nagarkar SS, Chaudhari AK, Ghosh SK (2012) Role of temperature on framework dimensionality: supramolecular isomers of Zn3(RCOO)8 based metal organic frameworks. Cryst Growth Des. https://doi.org/10.1021/cg201630c
Dikhtiarenko A, Serra-Crespo P, Castellanos S, Pustovarenko A, Mendoza-Meronõ R, Garcia-Granda S, Gascon J (2016) Temperature-dependent supramolecular isomerism of lutetium-aminoterephthalate metal–organic frameworks: synthesis crystallography and physical properties. Cryst Growth Des. https://doi.org/10.1021/acs.cgd.6b00274
Cheetham AK, Rao CN, Feller RF (2006) Structural diversity and chemical trends in hybrid inorganic–organic framework materials. Chem Commun. https://doi.org/10.1039/B610264F
Martí-Rujas J, Kawano M (2013) Kinetic products in coordination networks: ab initio x-ray powder diffraction analysis. Acc Chem Res. https://doi.org/10.1021/ar300212v
Bulavchenko AI, Yu PT, Demidova MG, Terzi EA, Beketova DI, Beisel NF (2020) The formation of Me(AOT)n micelles as nanoreactors, crystallizers, and charging agents: cation-exchange solvent extraction versus direct injection solubilization. Solvent Extr Ion Exch. https://doi.org/10.1080/07366299.2020.1733747
Pan A, Yan L, Ma X, Wu Y, Zhang Y, Zhou G, He L (2020) Strongly luminescent and highly stable core-shell suprastructures from in-situ growth of CsPbBr3 perovskite nanocrystals in multidentate copolymer micelles. J Alloys Compd. https://doi.org/10.1016/j.jallcom.2020.156102
Adıgüzel R, Taşcıoğlu S (2013) Micelle nano-reactors as mediators of water-insoluble ligand complexation with Cu(II) ions in aqueous medium. Chem Pap. https://doi.org/10.2478/s11696-012-0283-7
Petrosko SH, Johnson R, White H, Mirkin CA (2016) Nanoreactors: small spaces big implications in chemistry. J Am Chem Soc. https://doi.org/10.1021/jacs.6b05393
Vriezema DM, Aragones MC, Elemans JAAW, Cornelissen JJLM, Rowan AE, Nolte RJM (2005) Self-assembled nanoreactors. Chem Rev. https://doi.org/10.1021/cr0300688
Zhang W, Zhang Q, Dong F, Zhao Z (2013) The multiple effects of precursors on the properties of polymeric carbon nitride. Int J Photoenergy. https://doi.org/10.1155/2013/685038
Dyjak S, Kiciński W, Huczko A (2015) Thermite-driven melamine condensation to CxNyHz graphitic ternary polymers: towards an instant, large-scale synthesis of g-C3N4. J Mater Chem A. https://doi.org/10.1039/C5TA00201J
Braml NE, Schnick W (2013) New heptazine based materials with a divalent cation – Sr[H2C6N7O3]2·4H2O and Sr[HC6N7(NCN)3]·7H2O. Z Anorg Allg Chem. https://doi.org/10.1002/zaac.201200345
Ming L, Yue H, Xu L, Chen F (2014) Hydrothermal synthesis of oxidized g-C3N4 and its regulation of photocatalytic activity. J Mater Chem A. https://doi.org/10.1039/C4TA04041D
Liu S, Sun H, O’Donnell K, Ang HM, Tade MO, Wang S (2016) Metal-free melem/g-C3N4hybrid photocatalysts for water treatment. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2015.11.003
Wolf S, Alam N, Feldmann C (2015) δ-KNO3: synthesis and structure of a new modification of potassium nitrate. Z Anorg Allg Chem. https://doi.org/10.1002/zaac.201400538
Zheng YQ, Adam A (1994) Potassium trans-tetraaquadicarbonatonickelate(II), K2[Ni(CO3)2(H2O)4]. Acta Crystallogr. https://doi.org/10.1107/S0108270193012855
Werner PE, Eriksson L WestdahlM (1985)TREOR, a semi-exhaustive trial-and-error powder indexing program for all symmetries. J Appl Crystallogr. https://doi.org/10.1107/S0021889885010512
Visser JW (1969) A fully automatic program for finding the unit cell from powder data. J Appl Crystallogr. https://doi.org/10.1107/S0021889869006649
Zlokazov VB (1992) MRIAAU - a program for autoindexing multiphase polycrystals. J Appl Crystallogr. https://doi.org/10.1107/S0021889891009366
Zlokazov VB (1995) AUTOX - a program for autoindexing reflections from multiphase polycrystal. Comput Phys Commun. https://doi.org/10.1016/0010-4655(94)00151-Q
Pawley GS (1981) Unit-cell refinement from powder diffraction scans. J Appl Crystallogr. https://doi.org/10.1107/S0021889881009618
Zhukov SG, ChernyshevVV BEV, Sonneveld EJ, Schenk H (2001) Application of simulated annealing approach for structure solution of molecular crystals from X-ray laboratory powder data. Z Krist. https://doi.org/10.1524/zkri.216.1.5.18998
Bushmeleva AS, Tafeenko VA, Zakharov VN, Lobova AL, Aslanov LA (2019) Ammonium cyamelurates: synthesis and crystalline structures. Struct Chem. https://doi.org/10.1007/s11224-018-1187-0
Zlokazov VB, Chernyshev VV (1992) MRIA - a program for a full profile analysis of powder multiphase neutron-diffraction time-of-flight (direct and Fourier) spectra. J Appl Crystallogr. https://doi.org/10.1107/S0021889891013122
Dollase WA (1986) Correction of intensities for preferred orientation in powder diffractometry: application of the March model. J Appl Crystallogr. https://doi.org/10.1107/S0021889886089458
Popa NC (1998) The (hkl) Dependence of diffraction-line broadening caused by strain and size for all Laue groups in Rietveld refinement. J Appl Crystallogr. https://doi.org/10.1107/S0021889897009795
Groom CR, Allen FH (2014) The Cambridge structural database in retrospect and prospect. Angew Chem Int Ed. https://doi.org/10.1002/anie.201306438
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr. https://doi.org/10.1107/S0108767307043930
Brandenburg K (2000) DIAMOND Release 21d. Crystal Impact GbR, Bonn
Lake JA (1967) An iterative method of slit-correcting small angle X-ray data. Acta Cryst. https://doi.org/10.1107/S0365110X67002440
Guinier A, Fournet G (1955) Small-angle scattering of x-rays. Wiley, New York
Liu S, Sun H, O’Donnell K, Ang HM, Tade MO, Wang S (2016) Metal-free melem/g-C3N4 hybrid photocatalysts for water treatment. J Colloid Interface Sci. https://doi.org/10.1016/j.jcis.2015.11.003
Vinogradov AV, Vinogradov VV (2014) Low-temperature sol–gel synthesis of crystalline materials. RSC Adv. https://doi.org/10.1039/C4RA04454A
Du H, Amstad E (2020) Water: how does it influence the CaCO3 formation? Angew Chem Int Ed. https://doi.org/10.1002/anie.201903662
Funding
This work was supported by Russian Foundation for Basic Research (grant 20-08-00097) and by M.V. Lomonosov Moscow State University Program of Development. X-ray and SAXS investigation were performed using equipment of Center of Joint use of IPCE RAS.
Author information
Authors and Affiliations
Contributions
A. S. Isbjakowa, chemical syntheses and analyzes
V. V. Chernyshev, powder methods of crystal structure determinations
V. A. Tafeenko, single crystal methods of crystal structure determination
A. A. Shiryaev, small-angle X-ray scattering
I. K. Kudryavtsev, analysis of kinetic and thermodynamic control
L. A. Aslanov, setting a scientific problem and choosing objects of research, supervision of research, analysis of results, writing of manuscript
Corresponding author
Ethics declarations
Ethical approval
All ethical standards mentioned in Instructions for Authors are fulfilled.
Conflicts of interest
The authors have no conflicts of interest to declare that are relevant to the content of this article.
Consent to participate
All authors agreed with participation in research and publication of the results.
Consent to publish
All authors agreed with the content and all gave explicit consent to submit to Structural Chemistry Journal. All authors obtained consent from the responsible authorities at the institute/organization where the work has been carried out, before submission. All listed authors have approved the manuscript before submission, including the names and order of authors;
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
ESM 1
(DOCX 28 kb)
Rights and permissions
About this article
Cite this article
Isbjakowa, A.S., Chernyshev, V.V., Tafeenko, V.A. et al. Kinetic control of zinc cyamelurate crystal formations. Struct Chem 32, 719–729 (2021). https://doi.org/10.1007/s11224-020-01721-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01721-7