Abstract
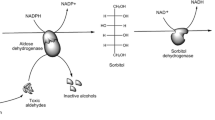
Aldehyde reductase (ALR1) and aldose reductase (ALR2) are both oxo-reductase enzymes of aldo-keto reductase (AKR) superfamily involved in several cellular processes. ALR1 plays an important role in colorectal cancer, lungs, and hepatic carcinoma, while ALR2 is involved in diabetic complications like retinopathy, neuropathy, and nephropathy cataract. Both the enzymes take part in distinct physiological processes, however, share more > 70% structural homology. This is the major cause behind the unachieved target selectivity of molecules that entered the development pipeline as ALR2 inhibitors. Chromene analogues have been widely explored for diverse biological activities, including antioxidant and diabetic complication prevention potential. For the identification of spatial fingerprints of target-specific chromene bearing ALR2 inhibitors over ALR1, Gaussian field-based comparative 3D QSAR models were generated on a dataset having ALR1 and ALR2 inhibitory activity. Both the ALR1 and ALR2 3D QSAR models were statistically fit with good predictive ability concerning PLS generated validation constraints. The comparative steric, electrostatic, hydrophobic, HBA, and HBD features were elucidated using respective contour maps for selective target specific favourable activity against ALR2 over ALR1. In addition, the five-point pharmacophores for ALR1 and ALR2 favourable features were also generated using the DHHRR_1 hypothesis for better insight on the distinctive features of ALR2 inhibitors compared to ALR1.





Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- 3D QSAR:
-
three-dimensional quantitative structural activity relationship
- ACCA:
-
acetyl-CoA Carboxylase-A
- AGEs:
-
advance glycation end products
- AKR:
-
aldo-keto reductase
- ALR1:
-
aldehyde reductase
- ALR2:
-
aldose reductase
- AM1:
-
Austin Model 1
- CNS:
-
central nervous system
- GSH:
-
glutathione
- HBA:
-
hydrogen bond acceptor
- HBD:
-
hydrogen bond donor
- MM2:
-
molecular mechanics
- MOPAC:
-
molecular orbital package
- PLS:
-
partial least square
- RMSD:
-
root mean square deviation
- RMSE:
-
root mean square error
- RNA:
-
ribonucleic acid
- ROS:
-
reactive oxygen species
- SD:
-
standard deviation
- SDH:
-
sorbitol dehydrogenase
References
International Diabetes Federation, IDF Diabetes Atlas Ninth edition (2019) https://www.diabetesatlas.org/upload/resources/material/20200302_133351_IDFATLAS9e-final-web.pdf. Accessed September 20, 2020
Verma SK, Thareja S (2017) Structure based comprehensive modelling, spatial fingerprints mapping and ADME screening of curcumin analogues as novel ALR2 inhibitors. PLoS One 12(4):e0175318
Ramana KV (2011) Aldose reductase: new insights for an old enzyme. BioMol Concepts 2(1–2):103–114
Khayami R, Hashemi SR, Kerachian MA (2020) Role of aldo-keto reductase family 1 member B1 (AKR1B1) in the cancer process and its therapeutic potential. J Cell Mol Med 24(16):8890–8902
Chen W-D, Zhang Y (2012) Regulation of aldo–keto reductases in human diseases. Front Pharmacol 3:35
Shaw N, Yang B, Millward A, Demaine A, Hodgkinson A (2014) AKR1B10 is induced by hyperglycaemia and lipopolysaccharide in patients with diabetic nephropathy. Cell Stress Chaperones 19(2):281–287
Alexiou P, Pegklidou K, Chatzopoulou M, Nicolaou I, Demopoulos VJ (2009) Aldose reductase enzyme and its implication to major health problems of the 21st century. Curr Med Chem 16(6):734–752
DiStefano JK, Davis B (2019) Diagnostic and prognostic potential of AKR1B10 in human hepatocellular carcinoma. Cancers 11(4):486
Griffin RJ, Fontana G, Golding BT, Guiard S, Hardcastle IR, Leahy JJ (2005) Selective benzopyranone and pyrimido [2, 1-a] isoquinolin-4-one inhibitors of dna-dependent protein kinase: synthesis, structure− activity studies, and radiosensitization of a human tumor cell line in vitro. J Med Chem 48(2):569–585
Makawana JA, Mungra DC, Patel MP, Patel RG (2011) Microwave assisted synthesis and antimicrobial evaluation of new fused pyran derivatives bearing 2-morpholinoquinoline nucleus. Bioorg Med Chem Lett 21(20):6166–6169
Ma T, Liu L, Xue H, Li L, Han C, Wang L (2008) Chemical library and structure–activity relationships of 11-demethyl-12-oxo calanolide A analogues as anti-HIV-1 agents. J Med Chem 51(5):1432–1446
Rambabu D, Mulakayala N, Kumar KR, Kumar GP, Mulakayala C, Kumar CS (2012) Synthesis and pharmacological evaluation of N-substituted 2-(2-oxo-2H-chromen-4-yloxy) propanamide as cyclooxygenase inhibitors. Bioorg Med Chem Lett 22(21):6745–6749
Nicolaides DN, Gautam DR, Litinas KE, Hadjipavlou-Litina DJ, Fylaktakidou KC (2004) Synthesis and evaluation of the antioxidant and antiinflammatory activities of some benzo [l] khellactone derivatives and analogues. Eur J Med Chem 39(4):323–332
Robert S, Bertolla C, Masereel B, Dogné J-M, Pochet L (2008) Novel 3-carboxamide-coumarins as potent and selective FXIIa inhibitors. J Med Chem 51(11):3077–3080
Abdelhafez OM, Amin KM, Ali HI, Abdalla MM, Batran RZ (2012) Synthesis of new 7-oxycoumarin derivatives as potent and selective monoamine oxidase A inhibitors. J Med Chem 55(23):10424–10436
Khanolkar AD, Lu D, Ibrahim M, Duclos J, Richard I, Thakur GA, Malan J, Phillip T (2007) Cannabilactones: a novel class of CB2 selective agonists with peripheral analgesic activity. J Med Chem 50(26):6493–6500
Heinrich T, Böttcher H, Schiemann K, Hölzemann G, Schwarz M, Bartoszyk GD (2004) Dual 5-HT1A agonists and 5-HT re-uptake inhibitors by combination of indole-butyl-amine and chromenonyl-piperazine structural elements in a single molecular entity. Bioorg Med Chem 12(18):4843–4852
Areias F, Costa M, Castro M, Brea J, Gregori-Puigjané E, Proença MF (2012) New chromene scaffolds for adenosine A2A receptors: synthesis, pharmacology and structure–activity relationships. Eur J Med Chem 54:303–310
Kesten SR, Heffner TG, Johnson SJ, Pugsley TA, Wright JL, Wise LD (1999) Design, synthesis, and evaluation of chromen-2-ones as potent and selective human dopamine D4 antagonists. J Med Chem 42(18):3718–3725
Piazzi L, Cavalli A, Belluti F, Bisi A, Gobbi S, Rizzo S (2007) Extensive SAR and computational studies of 3-{4-[(Benzylmethylamino) methyl] phenyl]-6, 7-dimethoxy-2 H-2-chromenone (AP2238) derivatives. J Med Chem 50(17):4250–4
Gopinath G, Sankeshi V, Alaparthi MD, Bandaru S, Pasala VK, Chittineni PR (2016) Design and synthesis of chiral 2H-chromene-N-imidazolo-amino acid conjugates as aldose reductase inhibitors. Eur J Med Chem 124:750–762
Costantino L, Rastelli G, Gamberini MC, Vinson JA, Bose P, Iannone A (1999) 1-Benzopyran-4-one antioxidants as aldose reductase inhibitors. J Med Chem 42(11):1881–1893
Ibrar A, Tehseen Y, Khan I, Hameed A, Saeed A, Furtmann N (2016) Coumarin-thiazole and-oxadiazole derivatives: synthesis, bioactivity and docking studies for aldose/aldehyde reductase inhibitors. Bioorg Chem 68:177–186
Varpe BD, Jadhav SB, Chatale BC, Mali AS, Jadhav SY, Kulkarni AA (2020) 3D-QSAR and pharmacophore modeling of 3, 5-disubstituted indole derivatives as Pim kinase inhibitors. Struct Chem 31:1675–1690
Kalva S, Vinod D, Saleena LM (2013) Field-and Gaussian-based 3D-QSAR studies on barbiturate analogs as MMP-9 inhibitors. Med Chem Res 22(11):5303–5313
Mali S, Chaudhari H (2019) Molecular modelling studies on adamantane-based Ebola virus GP-1 inhibitors using docking, pharmacophore and 3D-QSAR. SAR QSAR Environ Res 30(3):161–180
Yadav DK, Sharma P, Misra S, Singh H, Mancera RL, Kim K (2018) Studies of the benzopyran class of selective COX-2 inhibitors using 3D-QSAR and molecular docking. Arch Pharm Res 41(12):1178–1189
Thareja S, Rajpoot T, Verma SK (2015) Generation of comparative pharmacophoric model for steroidal 5α-reductase I and II inhibitors: a 3D-QSAR study on 6-azasteroids. Steroids 95:96–103
Verma SK, Thareja S (2016) Molecular docking assisted 3D-QSAR study of benzylidene-2, 4-thiazolidinedione derivatives as PTP-1B inhibitors for the management of Type-2 diabetes mellitus. RSC Adv 6(40):33857–33867
Verma SK, Thareja S (2016) Formylchromone derivatives as novel and selective PTP-1B inhibitors: a drug design aspect using molecular docking-based self-organizing molecular field analysis. Med Chem Res 25(7):1433–1467
Banjare L, Verma SK, Jain AK, Thareja S (2019) Structure guided molecular docking assisted alignment dependent 3D-QSAR study on steroidal aromatase inhibitors (SAIs) as anti-breast cancer agents. Lett Drug Des Discov 16(7):808–817
Acknowledgments
Niraj Kumar is grateful to All India Council for Technical Education (AICTE) for providing GPAT fellowship.
Author information
Authors and Affiliations
Contributions
Suresh Thareja conceptualized the present research. The computational methodology and formal analysis were performed by Niraj Kumar. The interpretation of all the results and original draft of manuscript was prepared by Sant Kumar Verma.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Verma, S.K., Kumar, N. & Thareja, S. Gaussian field-based comparative 3D QSAR modelling for the identification of favourable pharmacophoric features of chromene derivatives as selective inhibitors of ALR2 over ALR1. Struct Chem 32, 1289–1298 (2021). https://doi.org/10.1007/s11224-020-01714-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01714-6