Abstract
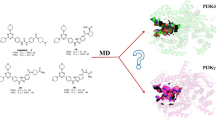
Phosphoinositide 3-kinase (PI3K) has been considered to be a potential drug target for the treatment of several human body diseases. Nowadays, great efforts have been made on the development of selective PI3Kδ inhibitors because of the FDA approval of idelalisib, which is the first listed PI3K inhibitor. But serious side effects occur during the use of idelalisib that greatly promotes the development of novel PI3Kδ inhibitors. Nevertheless, idelalisib is still an important milestone in the development of selective PI3Kδ inhibitors, but the detailed selective binding mechanisms between idelalisib and PI3Ks have not been well elucidated. Therefore, in this study, an integrated modeling strategy combining molecular docking, molecular dynamics simulation, and free energy calculation was performed to reveal the molecular-level binding mechanisms of idelalisib and class I PI3K. First, molecular docking was carried on to obtain a reasonable binding posture of idelalisib in different PI3K isoforms. Then, key residues for selective inhibition of PI3Kδ were highlighted by molecular dynamics simulation and energy calculations. Finally, idelalisib was also compared with its lead compound, IC87114, to reveal the reason for the higher potency of Idelalisib for PI3Kδ. We hope that this study would provide some guidance for the rational design of selective PI3Kδ inhibitors.

Graphical abstract




Similar content being viewed by others
References
Vanhaesebroeck B, Stephens L, Hawkins P (2012) PI3K signalling: the path to discovery and understanding. Nat Rev Mol Cell Biol 13(3):195–203. https://doi.org/10.1038/nrm3290
Zhu J, Hou T, Mao X (2015) Discovery of selective phosphatidylinositol 3-kinase inhibitors to treat hematological malignancies. Drug Discov Today 20(8):988–994. https://doi.org/10.1016/j.drudis.2015.03.009
Li K, Zhu J, Xu L, Jin J (2019) Rational design of novel phosphoinositide 3-kinase gamma (PI3Kgamma) selective inhibitors: a computational investigation integrating 3D-QSAR, molecular docking and molecular dynamics simulation. Chem Biodivers 16(7):e1900105. https://doi.org/10.1002/cbdv.201900105
Zhu J, Wang M, Cao B, Hou T, Mao X (2014) Targeting the phosphatidylinositol 3-kinase/AKT pathway for the treatment of multiple myeloma. Curr Med Chem 21(27):3173–3187. https://doi.org/10.2174/0929867321666140601204513
Knight ZA (2010) Small molecule inhibitors of the PI3-kinase family. Curr Top Microbiol Immunol 347:263–278. https://doi.org/10.1007/82_2010_44
Elmenier FM, Lasheen DS, Abouzid KAM (2019) Phosphatidylinositol 3 kinase (PI3K) inhibitors as new weapon to combat cancer. Eur J Med Chem 183:111718. https://doi.org/10.1016/j.ejmech.2019.111718
Wang X, Ding J, Meng LH (2015) PI3K isoform-selective inhibitors: next-generation targeted cancer therapies. Acta Pharmacol Sin 36(10):1170–1176. https://doi.org/10.1038/aps.2015.71
Zhang Z, Liu J, Wang Y, Tan X, Zhao W, Xing X, Qiu Y, Wang R, Jin M, Fan G, Zhang P, Zhong Y, Kong D (2018) Phosphatidylinositol 3-kinase beta and delta isoforms play key roles in metastasis of prostate cancer DU145 cells. FASEB J 32(11):5967–5975. https://doi.org/10.1096/fj.201800183R
Lannutti BJ, Meadows SA, Herman SE, Kashishian A, Steiner B, Johnson AJ, Byrd JC, Tyner JW, Loriaux MM, Deininger M, Druker BJ, Puri KD, Ulrich RG, Giese NA (2011) CAL-101, a p110delta selective phosphatidylinositol-3-kinase inhibitor for the treatment of B-cell malignancies, inhibits PI3K signaling and cellular viability. Blood 117(2):591–594. https://doi.org/10.1182/blood-2010-03-275305
Okkenhaug K, Burger JA (2016) PI3K signaling in normal B cells and chronic lymphocytic Leukemia (CLL). Curr Top Microbiol Immunol 393:123–142. https://doi.org/10.1007/82_2015_484
Nunes-Santos CJ, Uzel G, Rosenzweig SD (2019) PI3K pathway defects leading to immunodeficiency and immune dysregulation. J Allergy Clin Immunol 143(5):1676–1687. https://doi.org/10.1016/j.jaci.2019.03.017
Perry MWD, Abdulai R, Mogemark M, Petersen J, Thomas MJ, Valastro B, Westin Eriksson A (2019) Evolution of PI3Kgamma and delta inhibitors for inflammatory and autoimmune diseases. J Med Chem 62(10):4783–4814. https://doi.org/10.1021/acs.jmedchem.8b01298
Zhu J, Ke K, Xu L, Jin J (2019) Theoretical studies on the selectivity mechanisms of PI3Kdelta inhibition with marketed idelalisib and its derivatives by 3D-QSAR, molecular docking, and molecular dynamics simulation. J Mol Model 25(8):242. https://doi.org/10.1007/s00894-019-4129-x
Somoza JR, Koditek D, Villasenor AG, Novikov N, Wong MH, Liclican A, Xing W, Lagpacan L, Wang R, Schultz BE, Papalia GA, Samuel D, Lad L, McGrath ME (2015) Structural, biochemical, and biophysical characterization of idelalisib binding to phosphoinositide 3-kinase delta. J Biol Chem 290(13):8439–8446. https://doi.org/10.1074/jbc.M114.634683
Miller MS, Schmidt-Kittler O, Bolduc DM, Brower ET, Chaves-Moreira D, Allaire M, Kinzler KW, Jennings IG, Thompson PE, Cole PA, Amzel LM, Vogelstein B, Gabelli SB (2014) Structural basis of nSH2 regulation and lipid binding in PI3Kalpha. Oncotarget 5 (14):5198-5208. doi:10.18632/oncotarget.2263
Zhang X, Vadas O, Perisic O, Anderson KE, Clark J, Hawkins PT, Stephens LR, Williams RL (2011) Structure of lipid kinase p110beta/p85beta elucidates an unusual SH2-domain-mediated inhibitory mechanism. Mol Cell 41(5):567–578. https://doi.org/10.1016/j.molcel.2011.01.026
Shin Y, Suchomel J, Cardozo M, Duquette J, He X, Henne K, Hu YL, Kelly RC, McCarter J, McGee LR, Medina JC, Metz D, San Miguel T, Mohn D, Tran T, Vissinga C, Wong S, Wannberg S, Whittington DA, Whoriskey J, Yu G, Zalameda L, Zhang X, Cushing TD (2016) Discovery, optimization, and in vivo evaluation of benzimidazole derivatives AM-8508 and AM-9635 as potent and selective PI3Kdelta inhibitors. J Med Chem 59(1):431–447. https://doi.org/10.1021/acs.jmedchem.5b01651
Wang J, Wolf RM, Caldwell JW, Kollman PA, Case DA (2004) Development and testing of a general amber force field. J Comput Chem 25(9):1157–1174. https://doi.org/10.1002/jcc.20035
Berndt A, Miller S, Williams O, Le DD, Houseman BT, Pacold JI, Gorrec F, Hon WC, Liu Y, Rommel C, Gaillard P, Ruckle T, Schwarz MK, Shokat KM, Shaw JP, Williams RL (2010) The p110 delta structure: mechanisms for selectivity and potency of new PI(3)K inhibitors. Nat Chem Biol 6(2):117–124. https://doi.org/10.1038/nchembio.293
Case DA, Cheatham 3rd TE, Darden T, Gohlke H, Luo R, Merz Jr KM, Onufriev A, Simmerling C, Wang B, Woods RJ (2005) The Amber biomolecular simulation programs. J Comput Chem 26(16):1668–1688. https://doi.org/10.1002/jcc.20290
Stewart JJ (2004) Optimization of parameters for semiempirical methods IV: extension of MNDO, AM1, and PM3 to more main group elements. J Mol Model 10(2):155–164. https://doi.org/10.1007/s00894-004-0183-z
Stewart JJ (2013) Optimization of parameters for semiempirical methods VI: more modifications to the NDDO approximations and re-optimization of parameters. J Mol Model 19(1):1–32. https://doi.org/10.1007/s00894-012-1667-x
Zhu J, Wu Y, Xu L, Jin J (2020) Theoretical studies on the selectivity mechanisms of glycogen synthase kinase 3beta (GSK3beta) with pyrazine ATP-competitive inhibitors by 3DQSAR, molecular docking, molecular dynamics simulation and free energy calculations. Curr Comput Aided Drug Des 16(1):17–30. https://doi.org/10.2174/1573409915666190708102459
Zhu J, Li K, Xu L, Jin J (2019) Insight into the selective mechanism of phosphoinositide 3-kinase gamma with benzothiazole and thiazolopiperidine gamma-specific inhibitors by in silico approaches. Chem Biol Drug Des 93(5):818–831. https://doi.org/10.1111/cbdd.13469
Zhu J, Ke K, Xu L, Jin J (2019) Discovery of a novel phosphoinositide 3-kinase gamma (PI3Kγ) inhibitor against hematologic malignancies and theoretical studies on its PI3Kγ-specific binding mechanisms. RSC Advances 9(35):20207–20215. https://doi.org/10.1039/c9ra02649e
Xu L, Sun H, Li Y, Wang J, Hou T (2013) Assessing the performance of MM/PBSA and MM/GBSA methods. 3. The impact of force fields and ligand charge models. J Phys Chem B 117(28):8408–8421. https://doi.org/10.1021/jp404160y
Sun H, Li Y, Shen M, Tian S, Xu L, Pan P, Guan Y, Hou T (2014) Assessing the performance of MM/PBSA and MM/GBSA methods. 5. Improved docking performance using high solute dielectric constant MM/GBSA and MM/PBSA rescoring. Phys Chem Chem Phys 16(40):22035–22045. https://doi.org/10.1039/c4cp03179b
Wang E, Sun H, Wang J, Wang Z, Liu H, Zhang JZH, Hou T (2019) End-point binding free energy calculation with MM/PBSA and MM/GBSA: strategies and applications in drug design. Chem Rev 119(16):9478–9508. https://doi.org/10.1021/acs.chemrev.9b00055
Sun H, Duan L, Chen F, Liu H, Wang Z, Pan P, Zhu F, Zhang JZH, Hou T (2018) Assessing the performance of MM/PBSA and MM/GBSA methods. 7. Entropy effects on the performance of end-point binding free energy calculation approaches. Phys Chem Chem Phys 20(21):14450–14460. https://doi.org/10.1039/c7cp07623a
Xie T, Yu J, Fu W, Wang Z, Xu L, Chang S, Wang E, Zhu F, Zeng S, Kang Y, Hou T (2019) Insight into the selective binding mechanism of DNMT1 and DNMT3A inhibitors: a molecular simulation study. Phys Chem Chem Phys 21(24):12931–12947. https://doi.org/10.1039/c9cp02024a
Chohan TA, Chen JJ, Qian HY, Pan YL, Chen JZ (2016) Molecular modeling studies to characterize N-phenylpyrimidin-2-amine selectivity for CDK2 and CDK4 through 3D-QSAR and molecular dynamics simulations. Mol Biosyst 12(4):1250–1268. https://doi.org/10.1039/c5mb00860c
Bharadwaj VS, Dean AM, Maupin CM (2013) Insights into the glycyl radical enzyme active site of benzylsuccinate synthase: a computational study. J Am Chem Soc 135(33):12279–12288. https://doi.org/10.1021/ja404842r
Kong X, Sun H, Pan P, Tian S, Li D, Li Y, Hou T (2016) Molecular principle of the cyclin-dependent kinase selectivity of 4-(thiazol-5-yl)-2-(phenylamino) pyrimidine-5-carbonitrile derivatives revealed by molecular modeling studies. Phys Chem Chem Phys 18(3):2034–2046. https://doi.org/10.1039/c5cp05622e
Zhao S, Zhu J, Xu L, Jin J (2017) Theoretical studies on the selective mechanisms of GSK3beta and CDK2 by molecular dynamics simulations and free energy calculations. Chem Biol Drug Des 89(6):846–855. https://doi.org/10.1111/cbdd.12907
Hou T, Wang J, Li Y, Wang W (2011) Assessing the performance of the MM/PBSA and MM/GBSA methods. 1. The accuracy of binding free energy calculations based on molecular dynamics simulations. J Chem Inf Model 51(1):69–82. https://doi.org/10.1021/ci100275a
Xue W, Liu H, Yao X (2012) Molecular mechanism of HIV-1 integrase-vDNA interactions and strand transfer inhibitor action: a molecular modeling perspective. J Comput Chem 33(5):527–536. https://doi.org/10.1002/jcc.22887
Zhu J, Pan P, Li Y, Wang M, Li D, Cao B, Mao X, Hou T (2014) Theoretical studies on beta and delta isoform-specific binding mechanisms of phosphoinositide 3-kinase inhibitors. Mol Biosyst 10(3):454–466. https://doi.org/10.1039/c3mb70314b
Wei M, Wang X, Song Z, Jiao M, Ding J, Meng LH, Zhang A (2015) Targeting PI3Kdelta: emerging therapy for chronic lymphocytic leukemia and beyond. Med Res Rev 35(4):720–752. https://doi.org/10.1002/med.21341
Funding
The study was supported by the National Natural Science Foundation of China (No. 21807049), the Fundamental Research Funds of Changzhou Vocational Institute of Engineering (11130300117010), the Fundamental Research Funds for the Central Universities (JUSRP51703A), and the Top-notch Academic Programs Project of Jiangsu Higher Education Institutions (PPZY2015B146).
Author information
Authors and Affiliations
Contributions
Z.J., L.H., and J.J. developed the study concept and design. Z.H. and S.H. performed the modeling studies. Z.H., Y.L., and C.Y. carried out the data analysis. Z.J., Z.H., and Y.L. drafted the manuscript, C.Y., L.H., and J.J. approved the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jingyu Zhu, Haoer Zhang and Li Yu are Equivalent authors
Electronic supplementary materials
ESM 1
(DOCX 19 kb)
Rights and permissions
About this article
Cite this article
Zhu, J., Zhang, H., Yu, L. et al. Computational investigation of the selectivity mechanisms of PI3Kδ inhibition with marketed idelalisib: combined molecular dynamics simulation and free energy calculation. Struct Chem 32, 699–707 (2021). https://doi.org/10.1007/s11224-020-01643-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01643-4