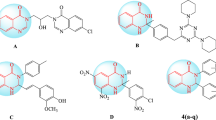
Abstract
In this work, a series of eight novel ring-substituted styrylquinolines were synthesized, and in silico physicochemical properties were estimated. The inhibitory activity of these compounds was evaluated in intracellular amastigotes of Leishmania (Viannia) panamensis, and their affinity for L. major dihydroorotate dehydrogenase DHODH (LmDHODH) and L. major tryparedoxin peroxidase TXNPx (LmTXNPx) was calculated by molecular docking, NCI index, and the recently developed IGM analysis, providing us useful insights about the forces governing the ligand-protein coupling. The eight synthesized molecules do not break the Lipinski, Ghose, Veber, Egan, and Muegge rules. Therefore, the bioavailability and absorption will not be poor.

Graphical abstract




Similar content being viewed by others
References
Ferreira LLG, Andricopulo AD (2018) Chemoinformatics strategies for leishmaniasis drug discovery. Front Pharmacol 9:1278. https://doi.org/10.3389/fphar.2018.01278
Bonardi A, Vermelho AB, Da Silva CV et al (2019) N-Nitrosulfonamides as carbonic anhydrase inhibitors: a promising chemotype for targeting Chagas disease and Leishmaniasis. ACS Med Chem Lett 10:413–418. https://doi.org/10.1021/acsmedchemlett.8b00430
Ochoa R, Watowich SJ, Flórez A et al (2016) Drug search for leishmaniasis: a virtual screening approach by grid computing. J Comput Aided Mol Des 30:541–552. https://doi.org/10.1007/s10822-016-9921-4
Bermúdez H, Rojas E, Garcia L et al (2006) Generic sodium stibogluconate is as safe and effective as branded meglumine antimoniate, for the treatment of tegumentary leishmaniasis in Isiboro Secure Park, Bolivia. Ann Trop Med Parasitol 100:591–600. https://doi.org/10.1179/136485906X118495
Layegh P, Yazdanpanah MJ, Vosugh EM et al (2007) Efficacy of azithromycin versus systemic meglumine antimoniate (glucantime) in the treatment of cutaneous leishmaniasis. Am J Trop Med Hyg 77:99–101. https://doi.org/10.4269/ajtmh.2007.77.99
Frézard F, Demicheli C, Ribeiro R (2009) Pentavalent antimonials: new perspectives for old drugs. Molecules 14:2317–2336. https://doi.org/10.3390/molecules14072317
Mackey TK, Liang BA, Cuomo R et al (2014) Emerging and reemerging neglected tropical diseases: a review of key characteristics. Risk Factors, and the Policy and Innovation Environment. https://doi.org/10.1128/CMR.00045-14
Barrett MP, Croft SL (2012) Management of trypanosomiasis and leishmaniasis. Br Med Bull 104:175–196
Guedes PMM, Silva GK, Gutierrez FRS, Silva JS (2011) Current status of Chagas disease chemotherapy. Expert Rev Anti-Infect Ther 9:609–620
El-Sayed MAA, El-Husseiny WM, Abdel-Aziz NI et al (2018) Synthesis and biological evaluation of 2-styrylquinolines as antitumour agents and EGFR kinase inhibitors: molecular docking study. J Enzyme Inhib Med Chem 33:199–209. https://doi.org/10.1080/14756366.2017.1407926
Richard JV, Werbovetz KA (2010) New antileishmanial candidates and lead compounds. Curr Opin Chem Biol 14:447–455
Delattin N, Bardiot D, Marchand A et al (2012) Identification of fungicidal 2,6-disubstituted quinolines with activity against candida biofilms. Molecules 17:12243–12251. https://doi.org/10.3390/molecules171012243
Afzal O, Kumar S, Haider MR et al (2015) A review on anticancer potential of bioactive heterocycle quinoline. Eur J Med Chem 97:871–910
Pálinkó I, Kukovecz Á, Török B, Körtvélyesi T (2000) On the mechanism of a modified Perkin condensation leading to α-phenylcinnamic acid stereoisomers - experiments and molecular modelling. Monatshefte fur Chemie 131:1097–1104. https://doi.org/10.1007/s007060070043
Pawar PM, Jarag KJ, Shankarling GS (2011) Environmentally benign and energy efficient methodology for condensation: an interesting facet to the classical Perkin reaction. Green Chem 13:2130–2134. https://doi.org/10.1039/c0gc00712a
Brindisi M, Brogi S, Relitti N et al (2015) Structure-based discovery of the first non-covalent inhibitors of Leishmania major tryparedoxin peroxidase by high throughput docking. Sci Rep 5:9705. https://doi.org/10.1038/srep09705
Coa JC, Castrillón W, Cardona W et al (2015) Synthesis, leishmanicidal, trypanocidal and cytotoxic activity of quinoline-hydrazone hybrids. Eur J Med Chem 101:746–753. https://doi.org/10.1016/j.ejmech.2015.07.018
Zouhiri F, Desmaële D, D’Angelo J et al (2001) HIV-1 replication inhibitors of the styrylquinoline class: incorporation of a masked diketo acid pharmacophore. Tetrahedron Lett 42:8189–8192. https://doi.org/10.1016/S0040-4039(01)01767-1
Escobar P, Matu S, Marques C, Croft SL (2002) Sensitivities of Leishmania species to hexadecylphosphocholine (miltefosine), ET-18-OCH3 (edelfosine) and amphotericin B. Acta Trop 81:151–157. https://doi.org/10.1016/S0001-706X(01)00197-8
Vageli DP, Doukas SG, Spock T, Sasaki CT (2018) Curcumin prevents the bile reflux-induced NF-κB-related mRNA oncogenic phenotype, in human hypopharyngeal cells. J Cell Mol Med 22:4209–4220. https://doi.org/10.1111/jcmm.13701
Daina A, Michielin O, Zoete V (2017) SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci Rep 7:1–13. https://doi.org/10.1038/srep42717
Cheleski J, Rocha JR, Pinheiro MP et al (2010) Novel insights for dihydroorotate dehydrogenase class 1A inhibitors discovery. Eur J Med Chem 45:5899–5909. https://doi.org/10.1016/j.ejmech.2010.09.055
Cordeiro AT, Feliciano PR, Pinheiro MP, Nonato MC (2012) Crystal structure of dihydroorotate dehydrogenase from Leishmania major. Biochimie 94:1739–1748. https://doi.org/10.1016/j.biochi.2012.04.003
Fiorillo A, Colotti G, Boffi A et al (2012) The crystal structures of the tryparedoxin-tryparedoxin peroxidase couple unveil the structural determinants of Leishmania detoxification pathway. PLoS Negl Trop Dis 6. https://doi.org/10.1371/journal.pntd.0001781
Trott O, Olson A (2010) AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization and multithreading. J Comput Chem 31:455–461. https://doi.org/10.1002/jcc.21334.AutoDock
Stewart JJP (2016) MOPAC
Stewart JJP (2007) Optimization of parameters for semiempirical methods V: modification of NDDO approximations and application to 70 elements. J Mol Model 13:1173–1213. https://doi.org/10.1007/s00894-007-0233-4
Dassault Systèmes BIOVIA (2017) Discovery Studio Modeling Environment
Sanner MF (1999) Python: a programming language for software integration and development. J Mol Graph Model 17:55–84. https://doi.org/10.1016/S1093-3263(99)99999-0
Gasteiger J, Marsili M (1980) Iterative partial equalization of orbital electronegativity-a rapid access to atomic charges. Tetrahedron 36:3219–3228. https://doi.org/10.1016/0040-4020(80)80168-2
Berman HM, Westbrook J, Feng Z et al (2000) The protein data bank. Nucleic Acids Res 28:235–242. https://doi.org/10.1093/nar/28.1.235
Madhavi Sastry G, Adzhigirey M, Day T et al (2013) Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J Comput Aided Mol Des 27:221–234. https://doi.org/10.1007/s10822-013-9644-8
Schrödinger Release 2020–1: Maestro, Schrödinger,... - Google Académico. https://scholar.google.com/scholar?hl=es&as_sdt=0%2C5&q=Schrödinger+Release+2020-1%3A+Maestro%2C+Schrödinger%2C+LLC%2C+New+York%2C+NY%2C+2020&btnG=. Accessed 17 Mar 2020
Johnson ER, Keinan S, Mori-Sánchez P et al (2010) Revealing noncovalent interactions. J Am Chem Soc 132:6498–6506. https://doi.org/10.1021/ja100936w
Contreras-García J, Johnson ER, Keinan S et al (2011) NCIPLOT: a program for plotting noncovalent interaction regions. J Chem Theory Comput 7:625–632. https://doi.org/10.1021/ct100641a
Humphrey W, Dalke A, Schulten K (1996) VMD: visual molecular dynamics. J Mol Graph 14:33–38
IGMPlot: a program revealing non-covalent interactions. http://igmplot.univ-reims.fr. Accessed 13 Jul 2020
Ponce-Vargas M, Lefebvre C, Boisson JC, Hénon E (2020) Atomic decomposition scheme of noncovalent interactions applied to host-guest assemblies. J Chem Inf Model 60:268–278. https://doi.org/10.1021/acs.jcim.9b01016
Luzina E, Chemistry AP-J of F, 2013 U (2013) Synthesis of 1-aroyl (1-arylsulfonyl)-4-bis (trifluoromethyl) alkyl semicarbazides as potential physiologically active compounds. Elsevier 148:41–48
Luzina EL, Popov AV (2014) Synthesis and anticancer activity evaluation of 3,4-mono- and bicyclosubstituted N-(het)aryl trifluoromethyl succinimides. J Fluor Chem 168:121–127. https://doi.org/10.1016/j.jfluchem.2014.09.019
Wang X, Lu K, Luo H et al (2018) In silico identification of small molecules as novel LXR agonists for the treatment of cardiovascular disease and cancer. J Mol Model 24:24–57. https://doi.org/10.1007/s00894-018-3578-y
Aly AA, Sayed SM, Abdelhafez ESM, Abdelhafez SMN, Abdelzaher WY, Raslan MA et al (2020) New quinoline-2-one/pyrazole derivatives; design, synthesis, molecular docking, anti-apoptotic evaluation, and caspase-3 inhibition assay. Bioorg Chem 94:103348
Chibli LA, Schmidt TJ, Nonato MC et al (2018) Natural products as inhibitors of Leishmania major dihydroorotate dehydrogenase. Eur J Med Chem 157:852–866. https://doi.org/10.1016/j.ejmech.2018.08.033
Piñeyro MD, Pizarro JC, Lema F et al (2005) Crystal structure of the tryparedoxin peroxidase from the human parasite Trypanosoma cruzi. J Struct Biol 150:11–22. https://doi.org/10.1016/j.jsb.2004.12.005
Ogungbe IV, Erwin WR, Setzer WN (2014) Antileishmanial phytochemical phenolics: molecular docking to potential protein targets. J Mol Graph Model 48:105–117. https://doi.org/10.1016/j.jmgm.2013.12.010
Wachsmuth LM, Johnson MG, Gavenonis J (2017) Essential multimeric enzymes in kinetoplastid parasites: a host of potentially druggable protein-protein interactions. PLoS Negl Trop Dis 11:1–16. https://doi.org/10.1371/journal.pntd.0005720
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 61 kb)
Rights and permissions
About this article
Cite this article
Cantero-López, P., Robledo Restrepo, S.M., Yañez, O. et al. Theoretical study of new LmDHODH and LmTXNPx complexes: structure-based relationships. Struct Chem 32, 167–177 (2021). https://doi.org/10.1007/s11224-020-01624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-020-01624-7