Abstract
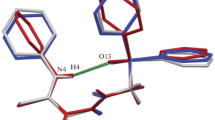
The four novel phosphonic acid analogues of phenylglycine with various substituents in phenyl ring (mostly fluorine atoms) have been synthesized by using procedure of amidoalkylation of phosphorus trichloride with aromatic aldehydes and acetamide. The NMR, ESI-MS spectroscopy, and single-crystal X-Ray diffraction methods were used to characterize unusual structures: the amino-(4-trifluoromethylbenzyl)-(1), amino-(3,4-difluorobenzyl)-(2), amino-(2,4,6-trifluorobenzyl)-(3), and amino-(2-fluoro-4-hydroxybenzyl)-(4) phosphonic acids. Since the α-aminophosphonates have a potential for biological activity and could be used as building blocks in medicinal chemistry, it is important to know their detail crystal structures and properties which, in turn, may extend the knowledge on their interaction with physiologic receptors.














Similar content being viewed by others
Change history
27 February 2020
Corrections are needed to the original version of this article.
References
Mastalerz P (1959). Arch Immun Ter Dośw 2:201–210
Mastalerz P (1960). Chem Abstr 54:6843
Mucha A, Kafarski P, Berlicki Ł (2011) Remarkable potential of the α-aminophosphonate/phosphinate structural motif in medicinal chemistry. J Med Chem 54:5955–5980
Mucha A (2012) Synthesis and Modifications of Phosphinic Dipeptide Analogues. Molecules 17:13530–13,568
Węglarz-Tomczak E, Vassiliou S, Mucha A (2016) Discovery of potent and selective inhibitors of human aminopeptidases ERAP1 and ERAP2 by screening libraries of phosphorus-containing amino acid and dipeptide analogues. Bioorg Med Chem Lett 26:4122–4126
Fang YL, Wu ZL, Xiao MW, Tang YT, Li KM, Ye J, Xiang JN, Hu AX (2016) One-Pot Three-Component Synthesis of Novel Diethyl((2-oxo-1,2-dihydroquinolin-3-yl)(arylamino)methyl)phosphonate as Potential Anticancer Agents. Int J Mol Sci 17:653–667
Bhattacharya AK, Raut DS, Rana KC, Polanki IK, Khan MS, Iram S (2013) Diversity-oriented synthesis of α-aminophosphonates: a new class of potential anticancer agents. Eur J Med Chem 66:146–152
Dake SA, Raut DS, Kharat KR, Mhaske RS, Deshmukh SU, Pawar RP (2011) Ionic liquid promoted synthesis, antibacterial and in vitro antiproliferative activity of novel α-aminophosphonate derivatives. Bioorg Med Chem Lett 21:2527–2532
Hellal A, Chafaa S, Chafai N, Touafri L (2017) Synthesis, antibacterial screening and DFT studies of series of α-amino-phosphonates derivatives from aminophenols. J Mol Struct 1134:217–225
Lan X, Xie D, Yin L, Wang Z, Chen J, Zhang A, Song B, Hu D (2017) Novel α,β-unsaturated amide derivatives bearing α-amino phosphonate moiety as potential antiviral agents. Bioorg Med Chem Lett 27:4270–4273
Romero-Estudillo I, Viveros-Ceballos JL, Cazares-Carreño O, González-Morales A, Flores de Jesús B, López-Castillo M, Razo-Hernández RS, Castañeda-Corral G, Ordóñez M (2018) Synthesis of new α-aminophosphonates: Evaluation as anti-inflammatory agents and QSAR studies. Bioorg Med Chem 15:2376–2386
Qin Y, Xing R, Liu S, Yu H, Li K, Hu L, Li P (2014) Synthesis and antifungal properties of (4-tolyloxy)-pyrimidyl-α-aminophosphonates chitosan derivatives. Int J Biol Macromol 63:83–91
Kafarski P, Lejczak B (2001) Aminophosphonic Acids of Potential Medical Importance. Curr Med Chem Anticancer Agents 1:301–312
Lejczak B, Kafarski P (2009) Biological Activity of Aminophosphonic Acids and Their Short Peptides. Top Heterocycl Chem 20:31–63
Wang J, Sanchez-Rosello M, Acena C, del Pozo JL, Sorochinsky AE, Fustero S, Soloshonok VA, Liu H (2014) Fluorine in pharmaceutical industry: fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem Rev 114:2432–2506
O’Hagan D, Rzepa HS (1997) Some influences of fluorine in bioorganic chemistry. Chem Commun 7:645–652
LeVine H (1999) Quantification of beta-sheet amyloid fibril structures with thioflavin T. Methods Enzymol 309:274–284
Lemal DM (2004) Perspective on Fluorocarbon Chemistry. J Org Chem 69:1–11
Ojima I (2009) Fluorine in Medicinal Chemistry and Chemical Biology, New York
Minks C, Huber R, Moroder L, Budisa N (2000) Noninvasive tracing of recombinant proteins with “fluorophenylalanine-fingers”. Anal Biochem 284:29–34
Wanat W, Talma M, Hurek J, Pawełczak M, Kafarski P (2018) Substituted phosphonic analogues of phenylglycine as inhibitors of phenylalanine ammonia lyase from potatoes. Biochimie 115:119–127
Oleksyszyn J, Soroka M, Rachoń J (1978) Phosphorus analogs of amino-acids and pepetides. 2. Phosphoanalogs and phosphinanalogs of cycloleucin. Chimia 32:253–255
Oleksyszyn J, Tyka R, Mastalerz P (1978) Direct synthesis of 1- aminoalkanephosphonic and 1- aminoalkanephosphinic acids from phosphorus trichloride or dichlorophosphines. Synthesis:479–480
Soroka M (1989) Comments on the synthesis of aminomethylphosphonic acid. Synthesis:547–548
CrysAlis CCD (2002) Oxford Diffraction Ltd.: Abingdon, England, CrysAlis RED (2002) Oxford Diffraction Ltd.: Abingdon, England
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Sheldrick GM (2015) Crystal structure refinement with SHELXL. Acta Crystallogr Sect C 71:3–8
Macrae CF, Bruno IJ, Chisholm JA, Edgington PR, McCabe P, Pidcock E, Rodriguez-Monge L, Taylor R, van de Streek J, Wood PA (2008) New Features for the Visualization and Investigation of Crystal Structures. J Appl Crystallogr 41:466–470
Sawka-Dobrowolska W (1985) Structure of α-(Isopropylamino)salicylphosphonic Acid Hemihydrate, C10H16NO4P.0.5H2O. Acta Crystallogr Sect C 41:84–86
Sawka-Dobrowolska W (1988) Structure of (R), 1-[(R)-1-Phenylethylamino]benzylphosphonic Acid Sesquihydrate. Acta Crystallogr Sect C 44:1624–1627
Nazir H, Yilmaz H, Tahir MN, Ülkü D (1999) Synthesis of Substituted α-N-(2-Hydroxyethyl)- Aminomethylphosphonic Acid Monoethylester Derivatives. Synth React In Met-Org Chem 29(10):1821–1828
Sowa M, Ślepokura K, Goldeman W, Duczmal M, Wojciechowska A, Matczak-Jon E (2013) Structural characterization of pyridin-2-, −3-, and − 4-yl functionalized (iminodimethanediyl)bis(phosphonic) acids: Insight into the cobalt(II) and copper(II) complexes of pyridin-2-yl derivative. Polyhedron 50:398–409
Hua F, Meijuan F, Xiaoxia L, Guo T, Yufen Z (2007) Syntheses, Characterizations, and Crystal Structures of Phosphonopeptides. Heteroat Chem 18:9–15
Naydenova ED, Todorov PT, Troev KD (2010) Recent synthesis of aminophosphonic acids as potential biological importance. Amino Acids 38:23–30
Visnjevac A, Tusek-Bozic L (2004) Two types of monoethyl a-anilino-benzylphosphonates: a zwitterion and a molecular compound. Acta Crystallogr Sect C 60:434–437
Tong F, Sun ZG, Chen K, Zhu YY, Wang WN, Jiao CQ, Wang CL, Li C (2011) Hydrothermal synthesis, structures, and luminescent properties of zinc(II) and cadmium(II) phosphonates with a 3D framework structure using terephthalate as second linkers. Dalton Trans 40:5059–5065
Spackman MA, McKinnon JJ (2002) Fingerprinting intermolecular interactions in molecular crystals. Cryst Eng Comm 4:378–392
Wolff SK, Grimwood DJ, McKinnon JJ, Turner MJ, Jayatilaka D, Spackman MA (2012) CrystalExplorer (Version 3.1). University of Western Australia
Funding
This work was supported by Narodowe Centrum Nauki, grant number 2017/26/M/ST5/00437.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wanat, W., Dziuk, B. & Kafarski, P. New crystal structures of fluorinated α-aminophosphonic acid analogues of phenylglycine. Struct Chem 31, 1197–1209 (2020). https://doi.org/10.1007/s11224-019-01483-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-019-01483-x