Abstract
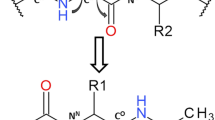
Solution structures of new pyrazine-based pseudotripeptide with amidoxime function and simplified pseudodipeptide analogs were determined by a combination of IR and NMR spectroscopic studies and molecular dynamic simulations using explicit chloroform as a solvent. It was found that proline-phenylalanine dipeptide residue and amidoxime moiety in o-position are essential for intramolecular hydrogen bonding including a seven-membered γ-turn formation. In addition, a cis/trans equilibrium study was present for prolyl amides in polar solvents (D2O and DMSO). A phenylalanine substituent was found to exhibit profound effect on thermodynamic parameters in prolyl peptides. The presence of intramolecular hydrogen bonds dramatically increases the amount of trans isomer in non-hydrogen-bonding CHCl3 and significantly favor cis isomer in hydrogen-bonding solvents such as DMSO and D2O. All molecules are not cytotoxic therefore they can be further studied in relation to potent biological activities.








Similar content being viewed by others
References
Fylaktakidou KC, Hadjipavlou-Litina DJ, Litinas KE, Varella EA, Nicolaides DN (2008) Recent developments in the chemistry and in the biological applications of amidoximes. Curr Pharm Des 14:1001–1047
Jakopin Z, Dolenc MS (2008) Recent advances in the synthesis of 1,2,4- and 1,3,4-oxadiazoles. Curr Org Chem 12:850–898
Pace A, Pierro P (2009) The new era of 1,2,4-oxadiazoles. Org Biomol Chem 7:4337–4348
Kumar D, Patel G, Chavers AK, Chang KH, Shah K (2011) Synthesis of novel 1,2,4-oxadiazoles and analogues as potential anticancer agents. Eur J Med Chem 46:3085–3092
Borg S, Estenne-Bouhtou G, Luthman K, Csoeregh I, Hesselink W, Hacksell U (1995) Synthesis of 1,2,4-oxadiazole-, 1,3,4-oxadiazole-, and 1,2,4-triazole-derived dipeptidomimetics. J Org Chem 60:3112–3120
Rehse K, Brehme F (1998) New NO donors with antithrombotic and vasodilating activities, part 26. Amidoximes and their prodrugs. Arch Pharm 331:375–379
Jousserandot A, Boucher JL, Henry Y, Niklaus B, Clement B, Mansuy D (1998) Microsomal cytochrome P450 dependent oxidation of N-hydroxyguanidines, amidoximes, and ketoximes: mechanism of the oxidative cleavage of their C = N(OH) bond with formation of nitrogen oxides. Biochemistry 37:17179–17191
Oresmaa L, Kotikoski H, Haukka M, Oksala O, Pohjala E, Vapaatalo H, Moilanen E, Vainiotalo P, Aulaskari P (2006) Synthesis, ocular effects, and nitric oxide donation of imidazole amidoximes. Eur J Med Chem 41:1073–1079
Clement B (2002) Reduction of N-hydroxylated compounds: amidoximes (N-hydroxyamidines) as pro-drugs of amidines. Drug Metab Rev 34:565–579
Ettmayer P, Amidon GL, Clement B, Testa B (2004) Lessons learned from marketed and investigational prodrugs. J Med Chem 47:2393–2404
Peterlin-Mašič L, Cesar J, Zega A (2006) Metabolism-directed optimisation of antithrombotics: the prodrug principle. Curr Pharm Des 12:73–91
Clement B, Lopian K (2003) Characterization of in vitro biotransformation of new, orally active, direct thrombin inhibitor ximelagatran, an amidoxime and ester prodrug. Drug Met Disp 31:645–651
Rautio J, Kumpulainen H, Heimbach T, Oliyai R, Oh D, Järvinen T, Savolainen J (2008) Prodrugs: design and clinical applications. Nat Rev Drug Discov 7:255–268
Wedemeyer WJ, Welker E, Scheraga HA (2002) Proline cis-trans isomerization and protein folding. Biochemistry 41:14637–14644
Beausoleil E, Lubell WD (1996) Steric effects on the amide isomer equilibrium of prolyl peptides. Synthesis and conformational analysis of N-acetyl-5-tert-butylproline N′-methylamides. J Am Chem Soc 118:12902–12908
Halab L, Lubell WD (2002) Effect of sequence on peptide geometry in 5-tert-butylprolyl type VI β-turn mimics. J Am Chem Soc 124:2474–2484
Taylor CM, Hardre R, Edwards PJB, Park JH (2003) Factors affecting conformation in proline-containing peptides. Org Lett 5:4413–4416
Jiménez AI, Cativiela C, Gómez-Catalán J, Pérez JJ, Aubry A, París M, Marraud M (2000) Influence of side chain restriction and NH···π interaction on the β-turn folding modes of dipeptides incorporating phenylalanine cyclohexane derivatives. J Am Chem Soc 122:5811–5821
Koopmanschap G, Ruijter E, Orru RVA (2014) Isocyanide-based multicomponent reactions towards cyclic constrained peptidomimetics. Beilstein J Org Chem 10:544–598
Gante J (1994) Peptidomimetics—tailored enzyme inhibitors. Angew Chem Int Ed Engl 33:1699–1720
Ovdiichuk OV, Hordiyenko OV, Medviediev VV, Shishkin OV, Arrault A (2015) Efficient synthesis of nicotinic acid based pseudopeptides bearing an amidoxime function. Synthesis 47:2285–2293
Ovdiichuk OV, Hordiyenko OV, Arrault A (2016) Synthesis and conformational study of novel pyrazine-based pseudopeptides bearing amidoxime, amidoxime ester and 1,2,4-oxadiazole units. Tetrahedron 72:3427–3435
Holenz J, Karlstroem S, Kihlstroem J, Kolmodin K, Lindstroem J, Rakos L, Rotticci D, Swahn BM, Von Berg S (2011) WO2011002409.
Ovdiichuk OV, Hordiyenko O, Voitenko Z, Arrault A, Medviediev V (2013) Methyl N-(3-cyano-picolino-yl)-l-tryptophanate. Acta Cryst E E69:o1810
Joshi KB, Verma S (2008) Sequence shuffle controls morphological consequences in a self-assembling tetrapeptide. J Pept Sci 14:118–126
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, van de Streek J (2006) Mercury: visualization and analysis of crystal structures. J Appl Crystallogr 39:453–457
Case DA, TAD, Cheatham TE, III, Simmerling CL, Wang J, Duke RE, Luo R, Crowley M, Walker RC, Zhang W, Merz KM, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Yang L, Tan C, Mongan J, Hornak V, Cui G, Mathews DH, Seetin MG, Sagui C, Babin V, Kollman PA (2008) AMBER 10, University of California, San Francisco.
Humbert-Voss E, Arrault A, Jamart-Grégoire B (2014) Synthesis and conformational behavior of pseudopeptides containing δ-azaproline. A cis conformational preference for Xaa1–δ-azaPro bond. Tetrahedron 70:363–370
Zhou Z, Deng C, Abbas C, Didierjean C, Averlant-Petit MC, Bodiguel J, Vanderesse R, Jamart-Gregoire B (2014) Synthesis and structural characterization of 2:1 [α/Aza]-oligomers. Eur J Org Chem 34:7643–7650
Fischer L, Didierjean C, Jolibois F, Semetey V, Lozano JM, Briand JP, Marraud M, Poteau R, Guichard G (2008) Propensity for local folding induced by urea fragment in short-chain oligomers. Org Biomol Chem 6:2596–2610
Dado GP, Gellman SH (1993) Structure and thermodynamic characterization of temperature-dependant changes in the folding pattern of a synthetic triamine. J Am Chem Soc 115:4228–4245
Chang X-W, Han Q-C, Jiao Z-G, Weng L-H, Zhang D-W (2010) 1-Aminoxymethylcyclopropanecarboxylic acid as building bloc of β N-O turn and helix: synthesis and conformaional analysis in solution and in the solid state. Tetrahedron 66:9733–9737
MacArthur MW, Thornton JM (1991) Influence of proline residues on protein conformation. J Mol Biol 218:397–412
Fischer S, Dunbrack RL, Karplus M (1994) Cis-trans imide isomerization of the proline dipeptide. J Am Chem Soc 116:11931–11937
Acknowledgements
The authors thank the NMR facilities SCBIM (Service Commun de Bioingénierie Moléculaire, Cellulaire et Thérapeutique), FR3209 CNRS-UL of Université de Lorraine, and X-Ray diffraction facilities of Université de Lorraine. We also thank Isabelle FRIES for cytocompatibility tests and to Olivier FABRE for NMR analysis and Emmanuel Wenger for XRD experiments.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
.
ESM 1
(DOCX 7725 kb)
Rights and permissions
About this article
Cite this article
Ovdiichuk, O., Hordiyenko, O., Fotou, E. et al. Conformational studies of new pseudotripeptide with pyrazine amidoxime motif and simplified analogs using IR, NMR spectroscopy, and molecular dynamic simulations. Struct Chem 28, 813–822 (2017). https://doi.org/10.1007/s11224-016-0870-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0870-2