Abstract
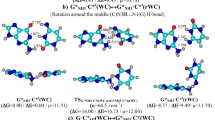
To elucidate the influence of solvating water molecules on the attacking mechanism of OH-radical to DNA base pairs (G–C and A–T), we investigated the reaction mechanism in water, by the use of the density functional theory (DFT) calculations by considering water molecules explicitly. The results reveal that OH-radical is stabilized near the NH2 group of cytosine of G–C by the water molecules hydrogen bonded to the OH-radical and that 2.5 kcal/mol activation free energy is needed for extracting the hydrogen atom from the NH2 group. On the other hand, OH-radical prefers to extract the hydrogen atom from the NH2 group of adenine in the solvated A–T. As for the tautomeric reaction of the base pair attacked by OH-radical, we found the transition state for the reaction from A to T to its tautomeric form A*–T*, although the activation free energy is rather large (25 kcal/mol). By contrast, in the G–C attacked by OH-radical, one central proton can move freely from G to C, resulting in the tautomeric form G*–C*. Therefore, our DFT calculations in explicit water molecules elucidate the possibility that the attacking of OH-radical to G–C causes its tautomeric form G*–C*, while A–T attacked by OH-radical cannot transform into its A*–T* form in a normal condition. This finding will be useful for predicting the effect of OH-radical on the genetic information recorded in DNA base sequences.













Similar content being viewed by others
References
Khanduri D, Collins S, Kumar A, Adhikary A, Sevilla MD (2008) J Phys Chem B 112:2168
Aydogan B, Bolch WE, Swarts SG, Turner JE, Marshall DT (1993) Radiat Res 169:223
Douki T, Cadet J (2008) In: Spotheim-Maurizot M, Mostafavi M, Douki T, Belloni J (eds) Radiation chemistry: from basics to applications in material and life sciences. EDP Sciences, Les Ulis Cedex A
Sies H (1993) FEBS J 215:213
Cooke MS, Evans MD, Dizdaroglu M, Lunce J (2003) FASEB J 17:1195
Cadet J, Douki T, Ravanat JL (2010) Free Radic Biol Med 49:9
Wagner JR, Cadet J (2010) Acc Chem Res 43:564
Tomita H, Kai M, Kusama T, Ito A (1998) Radiat Environ Biophys 36:235
Abolfath RM, van Duin ACT, Brabec T (2011) J Phys Chem A 115:11045
Kumar A, Pottiboyina V, Sevilla MD (2011) J Phys Chem B 115:15129
Cheng Q, Gu J, Compaan KR, Schaefer HF III (2010) Chem Eur J 16:11848
Mundy CJ, Colvin E, Quong AA (2002) J Phys Chem A 106:10063
Abolfath RM, Biswas PK, Rajnarayanam R, Brabec T, Kodym R, Papiez L (2012) J Phys Chem A 116:3940
Ceron-Carrasco JP, Jacquemin D (2012) RSC Adv 2:11867
Maruyama Y, Tachikawa M, Kawano S (2005) JSME Inter B48:196
Shimizu E, Hoshino R, Nomura K, Danilov VI, Kurita N (2013) J Mod Phys 4:422
Shimizu E, Tokuyama Y, Okutsu N, Nomura K, Danilov VI, Kurita N (2015) J Biomol Struct Dyn 33:158
Okutsu N, Shimamura K, Shimizu E, Shulga S, Danilov VI, Kurita N (2016) AIP Conf Proc 1709:020006
Hammer B, Hansen LB, Nørskov JK (1999) Phys Rev B 59:7413
Perdew J, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Delley B (1990) J Chem Phys 92:508
Delley B (2000) J Chem Phys 113:7756
Case DA (2012) AMBER12. University of California, San Francisco
Lindorff-Larsen K, Piana S, Palmo K, Maragakis P, Klepeis JL, Dror RO, Shaw DE (2010) Protein 78:1950
Jorgensen WL, Chandrasekhar J, Madura J, Klein ML (1983) J Chem Phys 79:926
Halgren TA, Lipscomb WN (1977) Chem Phys Lett 49:225
Acknowledgments
This study is supported by Open Partnership Joint Projects of JSPS Bilateral Joint Research Projects between Toyohashi University of Technology and the three institutes of National Academy of Science of Ukraine as well as the grants from the JSPS Grant-in-Aid for Challenging Exploratory Research (No. 22650061), the Murata Science Foundation, the Iketani Science and Technology Foundation, the Tatematsu Foundation and the CASIO Science Promotion Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shimamura, K., Okutsu, N., Shimizu, E. et al. Influence of solvating water molecules on the attacking mechanisms of OH-radical to DNA base pairs: DFT calculations in explicit waters. Struct Chem 27, 1793–1806 (2016). https://doi.org/10.1007/s11224-016-0800-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0800-3