Abstract
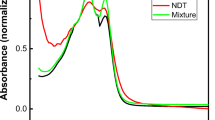
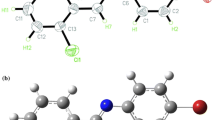
We design benzene-expanded Janus AT bases (J-AT1, J-AT2, BF1 and BF2) and investigate the structural and optical properties of these bases using DFT and TDDFT methods and also consider the effect of water solution and base pairing. The results show that J-AT1 has a nonplanar structure and its amino group is more asymmetric than the other three bases. J-AT1 and BF1 exist intramolecular H-bonds. The Janus AT base pairs exist three intermolecular H-bonds: NH···N has the largest energy, followed by NH···O and CH···O. The Janus AT base pairs retain the strength of H-bonds and maintain the stability of the base pair. The lowest absorption and emission wavelengths of the benzene-expanded Janus AT bases are all assigned to ππ* character arising from HOMO to LUMO transition, and they exhibit redshift due to the increase in effective conjugation length with the introduction of benzene. The excited state assignments in water solution are one-to-one correspondence to those in gas phase. The lowest absorption and emission wavelength of J-AT1 is blueshift, while J-AT2, BF1 and BF2 bases are redshift as compared to those in gas. The TD-B3LYP method predicts that the first excitation wavelengths of Janus AT base pairs are local excitation on the Janus AT base moieties, which is coincident with the result of CAM-B3LYP and M062X functional. Base pair can influence the excitation properties of base monomer. The results obtained from this theoretical investigation confirm that the position of benzene ring significantly influences the structure and optical properties of the Janus AT bases.






Similar content being viewed by others
References
Liu C, Martin CT (2001) Fluorescence characterization of the transcription bubble in elongation complexes of T7 RNA polymerase. J Mol Biol 308:465–475
Liu C, Martin CT (2002) Promoter Clearance by T7 RNA Polymerase initial bubble collapse and transcript dissociation monitored by base analog fluorescence. J Biol Chem 277:2725–2731
Zhang X, Wadkins RM (2009) DNA Hairpins Containing the Cytidine Analog Pyrrolo-dC: structural, thermodynamic, and spectroscopic studies. Biophys J 96:1884–1891
Buskiewicz IA, Burke JM (2012) Folding of the hammerhead ribozyme: pyrrolo-cytosine fluorescence separates core folding from global folding and reveals a pH-dependent conformational change. RNA 18:434–448
Wilhelmsson LM (2010) Fluorescent nucleic acid base analogues. Q Rev Biophys 43:159–183
Driscoll SL, Hawkins ME, Balis FM, Pfleiderer W, Laws WR (1997) Fluorescence properties of a new guanosine analog incorporated into small oligonucleotides. Biophys J 73:3277–3286
Hawkins ME, Pfleiderer W, Jungmann O, Balis FM (2001) Synthesis and fluorescence characterization of pteridine adenosine nucleoside analogs for DNA incorporation. Anal Biochem 298:231–240
Okamoto A, Saito Y, Saito I (2005) Design of base-discriminating fluorescent nucleosides. J Photochem Photobiol C 6:108–122
Okamoto A, Tainaka K, Saito I (2003) Clear distinction of purine bases on the complementary strand by a fluorescence change of a novel fluorescent nucleoside. J Am Chem Soc 125:4972–4973
Gao J, Liu H, Kool ET (2004) Expanded-size bases in naturally sized DNA: evaluation of steric effects in Watson-Crick pairing. J Am Chem Soc 126:11826–11831
Krueger AT, Kool ET (2008) Fluorescence of size-expanded DNA bases: reporting on DNA sequence and structure with an unnatural genetic set. J Am Chem Soc 130:3989–3999
Lee AH, Kool ET (2005) A new four-base genetic helix, yDNA, composed of widened benzopyrimidine-purine pairs. J Am Chem Soc 127:3332–3338
Liu H, Gao J, Kool ET (2005) Helix-forming properties of size-expanded DNA, an alternative four-base genetic form. J Am Chem Soc 127:1396–1402
Pan M-Y, Hang W, Zhao X-J, Zhao H, Deng P-C, Xing Z-H, Qing Y, He Y (2011) Janus-type AT nucleosides: synthesis, solid and solution state structures. Org Biomol Chem 9:5692–5702
Zhao H, Huang W, Wu X, Xing Z, He Y, Chen Q (2012) Different superstructures formed by Janus-type nucleosides. Chem Commun 48:6097–6099
Shin D, Tor Y (2011) Bifacial nucleoside as a surrogate for both T and A in duplex DNA. J Am Chem Soc 133:6926–6929
Song Q, Liu H, Liu J, Li Y, Wang H (2014) Theoretical study on absorption and emission spectra of size-expanded Janus-type AT nucleobases and effect of base pairing. Spectrochim Acta A Mol Biomol Spectrosc 121(2014):670–677
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648–5652
Lee C, Yang W, Parr RG (1988) Development of the colle-salvetti correlation-energy formula into a functional of the electron density. Phys Rev B 37:785–789
Brovarets OHO, Hovorun DM (2015) The nature of the transition mismatches with Watson-Crick architecture: the G*center dot T or G center dot T* DNA base mispair or both? A QM/QTAIM perspective for the biological problem. J Biomol Struct Dyn 33(2015):925–945
Brovarets OHO, Zhurakivsky RO, Hovorun DM (2014) Is the DPT tautomerization of the long A.G Watson-Crick DNA base mispair a source of the adenine and guanine mutagenic tautomers? A QM and QTAIM response to the biologically important question. J Comput Chem 35(2014):451–466
Zhurakivsky RO, Brovarets OO, Hovorun DM (2010) Is there adequate ionization mechanism of the spontaneous transitions? Quantum-chemical investigation. Biopolym Cell 26(5):398–405
Brovarets OHO, Hovorun DM (2015) The physicochemical essence of the purine center dot pyrimidine transition mismatches with Watson-Crick geometry in DNA: a center dot C* versa A*center dot C. A QM and QTAIM atomistic understanding. J Biomol Struct Dyn 33:28–55
Brovarets OHO, Hovorun DM (2015) Novel physico-chemical mechanism of the mutagenic tautomerisation of the Watson-Crick-like a center dot G and C center dot T DNA base mispairs: a quantum-chemical picture. Rsc Adv 5:66318–66333
Bader RFW (1990) Atoms in molecules: a quantum theory. Oxford University Press, Oxford
Brovarets OHO, Hovorun DM (2014) DPT tautomerisation of the G.A(syn) and A*.G*(syn) DNA mismatches: a QM/QTAIM combined atomistic investigation. Phys Chem Chem Phys 16:9074–9085
Kishor HB, Thorat G (2015) Ponnadurai Ramasami, Nagaiyan Sekar, NIR-emitting boradiazaindacene fluorophores -TD-DFT studies on electronic structure and photophysical properties. J Fluoresc 25:69–78
Qu Z-W, Zhu H, May V, Schinke R (2009) Time-dependent density functional theory study of the electronic excitation spectra of chlorophyllide a and pheophorbide a in solvents. J Phys Chem B 113:4817–4825
Varsano D, Garbesi A, Di Felice R (2007) Ab initio optical absorption spectra of size-expanded xDNA base assemblies. J Phys Chem B 111:14012–14021
Miertus S, Scrocco E (1981) Tomasi, electrostatic interaction of a solute with a continuum. A direct utilization of AB initio molecular potentials for the prevision of solvent effects. Chem Phys 55:117–129
Miertus S, Tomasi J (1982) Approximate evaluations of the electrostatic free energy and internal energy changes in solution processes. Chem Phys 65:239–245
Yanai T, Tew DP, Handy NC (2004) A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem Phys Lett 393:51–57
Zhao Y, Schultz NE, Truhlar DG (2006) Design of density functionals by combining the method of constraint satisfaction with parametrization for thermochemistry, thermochemical kinetics, and noncovalent interactions. J Chem Theory Comput 2:364–382
Trucks GW, Frisch MJ, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery Jr JA, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Keith T, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2010) Gaussian 09, revision B.01, Gaussian, Inc, Wallingford CT
Schonbohm J, Biegler-König F (2002) AIM2000, a program to analyze and visualize atoms in molecules, version 2.0. Bielefeld, Germany
Brovarets OHO, Yurenko YP, Hovorun DM (2014) Intermolecular CH center dot center dot center dot O/N H-bonds in the biologically important pairs of natural nucleobases: a thorough quantum-chemical study. J Biomol Struct Dyn 32:993–1022
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Mata I, Alkorta I, Espinosa E, Molins E (2011) Relationships between interaction energy, intermolecular distance and electron density properties in hydrogen bonded complexes under external electric fields. Chem Phys Lett 507:185–189
Brovarets OHO, Hovorun DM (2015) How many tautomerization pathways connect Watson-Crick-like G*center dot T DNA base mispair and wobble mismatches? J Biomol Struct Dyn 33:2297–2315
Iogansen AV (1999) Direct proportionality of the hydrogen bonding energy and the intensification of the stretching v(XH) vibration in infrared spectra. Spectrochim Acta A Mol Biomol Spectrosc 55:1585–1612
Nikolaienko TY, Bulavin LA, Hovorun DM (2012) Bridging QTAIM with vibrational spectroscopy: the energy of intramolecular hydrogen bonds in DNA-related biomolecules. Phys Chem Chem Phys 14:7441–7447
Leszczynski J (1992) Are the amino-groups in the nucleic-acid bases coplanar with the molecular rings? Ab initio HF/6-31G* and MP2/6-31G* studies. Int J Quantum Chem 19:43–55
Bader RFW (1998) A bond path: a universal indicator of bonded interactions. J Phys Chem A 102:7314–7323
Yang W, Parr RG (1994) Density-functional theory of atoms and molecules. Oxford University Press, Oxford, USA
Bondi A (1964) van der Waals volumes and radii. J Phys Chem 68(3):441–451
Yurenko YP, Zhurakivsky RO, Samijlenko SP, Hovorun DM (2011) Intramolecular CH center dot center dot center dot O hydrogen bonds in the AI and BI DNA-like conformers of canonical nucleosides and their Watson-Crick pairs. Quantum chemical and AIM analysis. J Biomol Struct Dyn 29:51–65
Wiberg KB, Bader RFW, Lau CDH (1987) Theoretical analysis of hydrocarbon properties. 2. Additivity of group properties and the origin of strain energy. J Am Chem Soc 109:1001–1012
Popelier PLA (1998) Characterization of a dihydrogen bond on the basis of the electron density. J Phys Chem A 102:1873–1878
Kodali G, Kistler KA, Narayanan M, Matsika S, Stanley RJ (2009) Change in electronic structure upon optical excitation of 8-vinyladenosine: an experimental and theoretical study. J Phys Chem A 114:256–267
Improta R, Barone V (2004) Absorption and fluorescence spectra of uracil in the gas phase and in aqueous solution: a TD-DFT quantum mechanical study. J Am Chem Soc 126:14320–14321
Kumar A, Sevilla MD (2006) Photoexcitation of dinucleoside radical cations: a time-dependent density functional study. J Phys Chem B 110:24181–24188
Brovarets OHO, Hovorun DM (2014) Can tautomerization of the A.T Watson-Crick base pair via double proton transfer provoke point mutations during DNA replication? A comprehensive QM and QTAIM analysis. J Biomol Struct Dyn 32:127–154
van Duijneveldt FB, van Duijneveldt-van de Rijdt JG, van Lenthe JH (1994) State of the art in counterpoise theory. Chem Rev 94:1873–1885
Volk DE, Thiviyanathan V, Somasunderam A, Gorenstein DG (2007) Ab initio base-pairing energies of an oxidized thymine product, 5-formyluracil, with standard DNA bases at the BSSE-free DFT and MP2 theory levels. Org Biomol Chem 5:1554–1558
Samanta PK, Manna AK, Pati SK (2012) Thieno analogues of RNA nucleosides: a detailed theoretical study. J Phys Chem B 116:7618–7626
Acknowledgments
Financial support from MOE & SAFEA for the 111 Project (B13025) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Liu, H., Song, Q. & Wang, H. Optical absorption and emission properties of benzene-expanded Janus AT nucleobase analogues: A DFT study. Struct Chem 27, 1175–1187 (2016). https://doi.org/10.1007/s11224-016-0743-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-016-0743-8