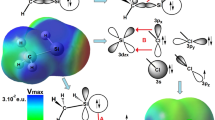
Abstract
Bonding intermolecular F···F interactions were analyzed in crystal of a fluorinated benzene derivative using topological analysis of experimental charge density distribution. It was found that the values of electron density and potential energy density in bond critical points of the F···F interactions in crystal depended on the distance between the fluorine atoms, rather than on the corresponding C–F···F angles. The directionality of these F···F interactions was demonstrated by DFT-D and MP2 calculations for model hexafluorobenzene dimers. Possible discrepancies between dimerization energies computed by ab initio methods and via empirical correlations are discussed.





Similar content being viewed by others
References
Metrangolo P, Neukirch H, Pilati T, Resnati G (2005) Halogen bonding based recognition processes: a world parallel to hydrogen bonding. Acc Chem Res 38:386–395
Rissanen K (2008) Halogen bonded supramolecular complexes and networks. CrystEngComm 10:1107
Reichenbächer K, Süss HI, Hulliger J (2005) Fluorine in crystal engineering—“the little atom that could”. Chem Soc Rev 34:22
Bader RFW (1990) Atoms in molecules—a quantum theory. Oxford University Press, Oxford
Bach A, Lentz D, Luger P (2001) Charge density and topological analysis of pentafluorobenzoic acid. J Phys Chem A 105:7405–7412
Hathwar VR, Guru Row TN (2011) Charge density analysis of heterohalogen (Cl···F) and homohalogen (F···F) intermolecular interactions in molecular crystals: importance of the extent of polarizability. Cryst Growth Des 11:1338–1346
Pavan MS, Durga Prasad K, Guru Row TN (2013) Halogen bonding in fluorine: experimental charge density study on intermolecular F···F and F···S donor–acceptor contacts. Chem Commun 49:7558
Levin VV, Dilman AD, Korlyukov AA et al (2007) Synthesis and structures of tris(pentafluorophenyl)silylamines. Russ Chem Bull 56:1394–1401
Osuna RM, Hernández V, Navarrete JTL et al (2011) Theoretical evaluation of the nature and strength of the F···F intermolecular interactions present in fluorinated hydrocarbons. Theor Chem Acc 128:541–553
Vasylyeva V, Shishkin OV, Maleev AV, Merz K (2012) Crystal structures of fluorinated pyridines from geometrical and energetic perspectives. Cryst Growth Des 12:1032–1039
Metrangolo P, Murray JS, Pilati T et al (2011) The fluorine atom as a halogen bond donor, viz. a positive site. CrystEngComm 13:6593
Bruker (2005) SAINT v7.23A
Sheldrick GM (2008) SADABS v2008/1, Bruker/Siemens Area Detector Absorption Correction Program. Bruker AXS, Madison
Sheldrick GM (2008) A short history of SHELX. Acta Crystallogr A 64:112–122
Hansen NK, Coppens P (1978) Testing aspherical atom refinements on small-molecule data sets. Acta Crystallogr A 34:909–921
Volkov A, Macchi P, Farrugia LJ, Gatti C, Mallinson P, Richter T, Koritsanszky T (2006) XD2006—a computer program package for multipole refinement, topological analysis of charge densities and evaluation of intermolecular energies from experimental and theoretical structure factors
Stash A, Tsirelson V (2002) WinXPRO: a program for calculating crystal and molecular properties using multipole parameters of the electron density. J Appl Crystallogr 35:371–373
Neese F (2012) The ORCA program system. Wiley Interdiscip Rev: Comput Mol Sci 2:73–78
Becke AD (1993) Density-functional thermochemistry. III. The role of exact exchange. J Chem Phys 98:5648
Grimme S, Ehrlich S, Goerigk L (2011) Effect of the damping function in dispersion corrected density functional theory. J Comput Chem 32:1456–1465
Weigend F, Ahlrichs R (2005) Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: design and assessment of accuracy. Phys Chem Chem Phys 7:3297
Klopper W, Manby FR, Ten-No S, Valeev EF (2006) R12 methods in explicitly correlated molecular electronic structure theory. Int Rev Phys Chem 25:427–468
Møller C, Plesset MS (1934) Note on an approximation treatment for many-electron systems. Phys Rev 46:618–622
Peterson KA, Adler TB, Werner H-J (2008) Systematically convergent basis sets for explicitly correlated wavefunctions: the atoms H, He, B–Ne, and Al–Ar. J Chem Phys 128:084102
Yousaf KE, Peterson KA (2008) Optimized auxiliary basis sets for explicitly correlated methods. J Chem Phys 129:184108
Espinosa E, Molins E, Lecomte C (1998) Hydrogen bond strengths revealed by topological analyses of experimentally observed electron densities. Chem Phys Lett 285:170–173
Lyssenko KA (2012) Analysis of supramolecular architectures: beyond molecular packing diagrams. Mendeleev Commun 22:1–7
Konovalova IS, Nelyubina YV, Lyssenko KA et al (2011) Intra- and intermolecular interactions in the crystals of 3,4-diamino-1,2,4-triazole and Its 5-methyl derivative. Experimental and theoretical investigations of charge density distribution. J Phys Chem A 115:8550–8562
Frolova NG, Savin ED, Goryunov EI et al (2010) Addition of bis(pentafluorophenyl)phosphinous acid to compounds with activated C=C bond as a method for the synthesis of first tertiary P, P-bis(pentafluorophenyl)phosphine oxides. Dokl Chem 430:18–23
Nicholson BK, Thwaite SE (2003) Tris(pentafluorophenyl)phosphine oxide. Acta Crystallogr Sect E Struct Rep Online 59:o1700–o1701
Shorafa H, Mollenhauer D, Paulus B, Seppelt K (2009) The two structures of the hexafluorobenzene radical Cation C6 F6 +. Angew Chem Int Ed 48:5845–5847
Schomaker V, Trueblood KN (1968) On the rigid-body motion of molecules in crystals. Acta Crystallogr B 24:63–76
Chęcińska L, Mebs S, Hübschle CB et al (2006) Reproducibility and transferability of topological data: experimental charge density study of two modifications of l-alanyl-l-tyrosyl-l-alanine. Org Biomol Chem 4:3242
Pendás AM, Francisco E, Blanco MA, Gatti C (2007) Bond paths as privileged exchange channels. Chem Eur J 13:9362–9371
Acknowledgments
This study was financially supported by Russian Science Foundation (Project 14-13-00884).
Author information
Authors and Affiliations
Corresponding author
Additional information
Dedicated to the memory of Prof. Oleg V. Shishkin.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Karnoukhova, V.A., Fedyanin, I.V. & Lyssenko, K.A. Directionality of intermolecular C–F···F–C interactions in crystals: experimental and theoretical charge density study. Struct Chem 27, 17–24 (2016). https://doi.org/10.1007/s11224-015-0622-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-015-0622-8