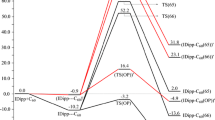
Abstract
The present article reports density functional studies on the Group 15 analogues of N-heterocyclic carbene (NHC) on their structure, reactivity, stability and ligating properties. Long-range corrected density functionals have been used due to its recent success in predicting orbital energies. These ligands are found to have greater π-accepting ability than NHC. Electron-donating substituents have a dramatic effect on their stability as well as ligating properties. Furthermore, natural resonance theory (NRT) calculations have been performed to determine the percentage weighting of resonance contributing structures. In addition, density-based global reactivity descriptors such as chemical potential, hardness, electrophilicity index and softness are calculated using four different density functional methods, and compared with CCSD(T) results. Moreover, the density-based local reactivity descriptors are employed to study the reactivity of Group 15 analogues. From the plot of dual descriptors, it is found that the “ene” centre of the Group 15 analogues of NHC is pseudodual.







Similar content being viewed by others
References
Arduengo AJ III, Harlow RL, Kline M (1991) J Am Chem Soc 113:361–363
Melaimi M, Soleilhavoup M, Bertrand G (2010) Angew Chem Int Ed 49:8810–8849
Hahn FE, Jahnke MC (2008) Angew Chem Int Ed 47:3122–3172
Segawa Y, Yamashita M, Nozaki K (2006) Science 314:113–115
Segawa Y, Yamashita M, Nozaki K (2009) J Am Chem Soc 131:9201–9203
Schmidt ES, Jockisch A, Schmidbaur H (1999) J Am Chem Soc 121:9758–9759
Yoo H, Carroll PJ, Berry DH (2006) J Am Chem Soc 128:6038–6039
Herrmann WA, Denk M, Behm J, Scherer W, Klingan FR, Bock H, Solouki B (1992) Wagner. Angew Chem Int Ed Engl 31:1485–1488
Pan B, Xu Z, Bezpalko MW, Foxman BM, Thomas CM (2012) Inorg Chem 51:4170–4179
Day GS, Pan B, Kellenberger DL, Foxman BM, Thomas CM (2011) Chem Commun 47:3634–3636
Heims F, Pfaff FF, Abram SLL, Farquhar ER, Bruschi M, Greco C, Ray K (2014) J Am Chem Soc 136:582–585
Tulchinsky Y, Iron MA, Botoshansky M, Gandelman M (2011) Nat Chem 3:525–531
Carmalt CJ, Lomeli V, McBurnett BG, Cowley AH (1997) Chem Commun 21:2095–2096
Caputo CA, Jennings MC, Tuononen HM, Jones ND (2009) Organometallics 28:990–1000
Denk MK, Gupta S, Ramachandran R (1996) Tetrahedron Lett 37:9025–9028
Burck S, Daniels J, Gans-Eichler T, Gudat D, Nättinen K, Nieger M (2005) Z Anorg Allg Chem 631:1403–1412
Denk MK, Gupta S, Lough AJ (1999) Eur J Inorg Chem 1999:41–49
Boche G, Andrews P, Harms K, Marsch M, Rangappa KS, Schimeczek M, Willeke C (1996) J Am Chem Soc 118:4925–4930
Gudat D, Haghverdi A, Hupfer H, Nieger M (2000) Chem Eur J 6:3414–3425
Burck S, Gudat D, Nieger M, Benkö Z, Nyulászi L, Szieberth D (2009) Z Allg Anorg Chem 635:245–252
Gudat D (2010) Acc Chem Res 43:1307–1316
Gudat D, Haghverdi A, Nieger M (2000) Angew Chem Int Ed 39:3084–3086
Schmid D, Loscher S, Gudat D, Bubrin D, Hartenbach I, Schleid T, Benkö Z, Nyulászi L (2009) Chem Commun 2009:830–832
Burck S, Götz K, Kaupp M, Nieger M, Weber J (2009) Schmedt auf der Günne J, Gudat. J Am Chem Soc 131:10763–10774
Benkö Z, Burck S, Gudat D, Hofmann M, Lissner F, Nyulászi L, Zenneck U (2010) Chem Eur J 16:2857–2865
Gudat D, Kaaz M, Bender J, Förster D, Frey W, Nieger M (2013) Dalton Trans 43:680–689
Mourgas G, Nieger M, Förster D, Gudat D (2013) Inorg Chem 52:4104–4122
Wilson DJD, Couchman SA, Dutton JL (2012) Inorg Chem 51:7657–7668
Tuononen MH, Roesler R, Dutton JL, Ragogna PJ (2007) Inorg Chem 46:10693–10706
Choudhury J (2011) Angew Chem Int Ed 50:10772–10774
Alcarazo M, Stork T, Anoop A, Thiel W, Fürstner A (2010) Angew Chem Int Ed 49:2542–2546
Guha AK, Sarmah S, Phukan AK (2010) Dalton Trans 39:7374–7383
Hohenberg P, Kohn W (1964) Phys Rev 136:864–871
Parr RG, Yang W (1989) Density functional theory for atoms and molecules. Oxford University Press, New York
Parr RG, Donnelly RA, Levy M, Palke WE (1978) J Chem Phys 68:3801–3807
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512–7516
Yang W, Parr RG (1985) Proc Natl Acad Sci USA 82:6723–8726
Koopmans TA (1933) Physica 104:1–6
Szabo A, Ostlund NS (1996) Modern quantum chemistry introduction to advanced electronic structure theory. Dover Publication, Inc, Mineola, New York
Parr RG, Yang W (1984) J Am Chem Soc 106:4049–4050
Yang W, Mortier WJ (1986) J Am Chem Soc 108:5708–5711
Morell C, Grand A, Toro-Labbé A (2005) J Phys Chem A 109:205–212
Cárdenas C, Rabi N, Ayers PW, Morell C, Jaramillo P, Fuentealba P (2009) J Phys Chem A 113:8660–8667
Parr RG, Szentpaly LV, Liu S (1999) J Am Chem Soc 121:1922–1924
Chattaraj PK, Maiti B, Sarkar U (2003) J Phys Chem A 107:4973–4975
Becke AD (1993) J Chem Phys 98:5648–5652
Becke AD (1988) Phys Rev A 38:3098–3100
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:785–789
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–654
McLean AD, Chandler GS (1980) J Chem Phys 72:5639–5648
Iikura H, Tsuneda T, Yanai T, Hirao K (2001) J Chem Phys 115:3540–3544
Yanai T, Tew DP, Handy NC (2004) Chem Phys Lett 393:51–57
Chai JD, Gordon MH (2008) Phys Chem Chem Phys 10:6615–6620
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347–1363
Neese F (2008) ORCA-An ab initio, Density functional and semiempirical program package, version 3.0–35, University of Bonn, Bonn
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Landis CR, Weinhold F (2013) NBO 6.0, Theoretical chemistry Institute, University of Wisconsin, Madison
Dixon DA, Arduengo AJ III (2006) J Phys Chem A 110:1968–1974
Dixon DA, Gutowski M (2005) J Phys Chem A 109:5129–5135
Nyulászi L, Veszprémi T, Forró A (2000) Phys Chem Chem Phys 2:3127–3129
Kar R, Song JW, Hirao K (2013) J Comp Chem 34:958–964
Glendening ED, Weinhold F (1998) J Comput Chem 19:593–609
Glendening ED, Weinhold F (1998) J Comput Chem 19:610–627
Glendening ED, Badenhoop JK, Weinhold F (1998) J Comput Chem 19:628–646
Eichler TG, Gudat D, Nättinen K, Neiger M (2006) Chem Eur J 12:1162–1173
Petz W, Kutschera C, Heitbaum M, Frenking G, Tonner R, Neumüller B (2005) Inorg Chem 44:1263–1274
Tonner R, Frenking G (2008) Chem Eur J 14:3260–3272
Tonner R, Frenking G (2008) Chem Eur J 14:3273–3289
Tonner R, Frenking G (2009) Organometallics 28:3901–3905
Tonner R, Frenking G (2009) Pure Appl Chem 81:597–614
Patel DS, Bharatam PV (2009) Chem Commun 2009:1064–1066
Geerlings P, Proft FD, Langenaeker W (2003) Chem Rev 103:1793–1874
Chattaraj PK, Sarkar U, Roy DR (2006) Chem Rev 106:2065–2091
Geerlings P, Proft FD (2008) Phys Chem Chem Phys 10:3028–3042
Oláh J, Proft FD, Veszprémi T, Geerlings P (2005) J Phys Chem A 109:1608–1615
Oláh J, Veszprémi T, Proft FD, Geerlings P (2007) J Phys Chem A 111:10815–10823
Correa JV, Jaque P, Oláh J, Labbé AT, Geerlings P (2009) Chem Phys Lett 470:180–186
Kelemen Z, Hollóczki O, Oláh J, Nyulászi L (2013) RSC Adv 3:7970–7978
Tsuneda T, Song JW, Suzuki S, Hirao K (2010) J Chem Phys 133:174101–174109
Sjoberg P, Murray JS, Brink T, Politzer P (1990) Can J Chem 68:1440–1443
Politzer P, Murray JS, Bulat FA (2010) J Mol Model 16:1731–1742
Bulat FA, Burgess JS, Matis BR, Baldwin JW, Macaveiu L, Murray JS, Politzer P (2012) J Phys Chem A 116:8644–8652
Kulshrestha P, Sukumar N, Murray JS, Giese RF, Wood TD (2009) J Phys Chem A 113:756–766
Acknowledgments
This work is dedicated to Dr. Sourav Pal on his 60th birthday. M. P. B thanks Council of Industrial and Scientific Research (CSIR), New Delhi, for financial assistance. RK thanks the SERB, Department of Science and Technology (DST), New Delhi for financial support [SB/FT/CS-132/2013]. The authors thank Netrakamal Bora for providing the optimized geometries for 2 and 3 with substituents CN, OH, NH 2 and OMe only.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11224_2014_552_MOESM1_ESM.doc
Figures S1–S2, Tables S1–S2 and Cartesian coordinates of all the molecules are provided in supporting information. (DOC 2715 kb)
Rights and permissions
About this article
Cite this article
Borpuzari, M.P., Guha, A.K. & Kar, R. Structural, electronic and reactivity studies on group 15 analogues of N-heterocyclic carbene. Struct Chem 26, 859–871 (2015). https://doi.org/10.1007/s11224-014-0552-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-014-0552-x