Abstract
Molecular orbital closed loops theory was used to explain the mechanism of the third-order nonlinear optical (NLO) response of polyanion cage structure of polyoxometalate by TDDFT study. The third-order NLO properties of three octa-polyoxometalate anion clusters of VIB group metals, β-[Cr8O26]4−, β-[Mo8O26]4−, and β-[W8O26]4−, were studied by DFT/TDDFT method systematically. The geometric structures of β-[Cr8O26]4− and β-[W8O26]4− were separately simulated by DFT method, based on the crystallographically determined geometry of the β-[Mo8O26]4− ion, and the thermo stability, and the second static hyperpolarizabilities, γ iiii , γ iijj , and γmean were calculated by finite-field method as an extension of the usual DFT energy run, and the results suggest that with no ligands coordinated, all of the three clusters possess moderately large hyperpolarizabilities, and the molybdate ion possess much larger NLO response than the chromate and the tungstate ions. In order to find out the reason of the big difference in the hyperpolarizabilities of the three polyoxometalates of the same group metals, the electronic properties were also studied by DFT method for the discussion of the origination of the NLO response, specially, the mechanism of the electronic structure change affecting the NLO response is analyzed and exhibited by the molecular orbital “closed loops” theory which has usually been used to estimate the thermo stability and redox ability of the polyoxometalate anion cages.
Graphical abstract
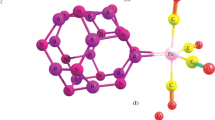
Without the coordination by any ligands, polyanion [M8O26]4− (M = Cr, Mo, W) in high symmetry structures present moderately high third-order NLO response by TDDFT study, even higher than the one-dimensional metal–metal bond compounds Cr(II)4, Mo(II)4, and some C60 derivatives. A new thought of molecular “orbital loops” for the theoretical exploration of the mechanism of NLO response were introduced, and applied to the polyanions and proved to be an effective analyzing method

.






Similar content being viewed by others
References
Pope MT (1983) Heteropoly and isopoly oxometalates. Springer-Verlag, Heidelberg
Pope MT, Muller A (1991) Angew Chem Int Ed Engl 30:34
Baker LCW, Glick DC (1998) Chem Rev 98:3
Pope MT, Müller A (1994) Polyoxometalates: from platonic solids to anti-retroviral activity. Kluwer, Dordrecht
Rhule JT, Hill CL, Judd DA (1998) Chem Rev 98:327
Kortz U, Hussain F, Reicke M (2005) Angew Chem Int Ed 44:3773
Mal SS, Kortz U (2005) Angew Chem Int Ed 44:3777
Yang Y, Cao MH, Hu CW, Guo YH, Wang EB (2004) J Nanosci Nanotechnol 4:833
Gregoriou VG, Rodman SE, Nair BR, Hammond PT (2002) J Phys Chem 106:11108
Sasic S, Clark DA, Mitchell JC, Snowden MJ (2005) Appl Spectrosc 59:630
Niven ML, Cruywagen JJ, Heyns JBB (2007) J Chem Soc Dalton Trans 1991
Xu JQ, Wang RZ, Yang GY, Xing YH, Li DM, Bu WM et al (1999) Chem Commun 11:983
Coe BJ, Jones LA, Harris JA, Sanderson EE (2003) Dalton Trans 2335
Zhang LH, Wang Y, Ma F, Liu CG (2012) J Organomet Chem 716:245
Janjua MRSA, Khan MU, Bashir B, Iqbal MA, Song YZ, Naqvi SAR, Khan ZA (2012) Comput Theor Chem 994:34
Li FJ, Hu XJ, Sa RJ (2013) Mol Phys (in press)
Koudoumas E, Konstantaki M, Mavromanolakis A, Couris S, Fanti M, Zerbetto F, Kordatos K, Prato M (2003) Chem Eur J 9:1529
Huo YP, Zeng HP, Jiang HF (2006) Chin J Organ Chem 26:1657
Nomiya K, Miwa M (1984) Polyhedron 3:341
Bridgeman AJ, Cavigliasso G (2002) Inorg Chem 41:3500
Bridgeman AJ (2002) J Phys Chem A 106:12151
Buckingham AD (1959) J Chem Phys 30:1580
Calaminici P, Jug K, Köster AM (1998) J Chem Phys 109:7756
Williams GRJ (1987) J Mol Struct Theochem 151:215
Fonseca Guerra C, Visser O, Snijders JG, te Velde G, Baerends EJ (1995) In: Clementi E, Corongiu C (eds) Methods and techniques for computational chemistry. STEF, Calgary, p 303
van Lenthe E, Baerends EJ, Snijders JG (1993) J Chem Phys 99:4597
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865
Schaefer CHuber, Ahlrichs R (1994) J Chem Phys 100:5829
Stephens PJ, Devlin FJ, Chabalowski CF, Frisch MJ (1994) J Phys Chem 98:11623
Becke AD (1993) J Chem Phys 98:5648
Cundari TR, Stevens WJ (1993) J Chem Phys 98:5555
Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F (2001) Theoretical Chemistry Institute. University of Wisconsin, Madison
Tytko KH, Mehmke J, Fischer S (1999) Struct Bond (Berlin) 93:129
Fukui H, Nakano M, Champagne B (2012) Chem Phys Lett 527:11
Lascola R, Wright JC (1997) Chem Phys Lett 269:79
Tytko KH, Mehmke J, Fischer S (1999) Struct Bond (Berlin) 93:129
Göller AH, Erhardt S, Grummt UW (2002) J Mol Struct Theochem 585:143
Acknowledgments
This work has been supported by National Natural Science Foundation of China (21277093), Liaoning BaiQianWan Talents Program (2010921004), Natural Science Foundation of Liaoning Province of China (201102156), and Program for Liaoning Excellent Talents in University (LR2011034). The authors also acknowledge the computer facility in the Virtual Laboratory for Computational Chemistry and Supercomputing Center of CNIC in Beijing.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, F., Hu, X., Sa, R. et al. Molecular orbital closed loops analysis of the third-order NLO response of polyanion [M8O26]4− (M = Cr, Mo, W): a TDDFT study. Struct Chem 25, 539–549 (2014). https://doi.org/10.1007/s11224-013-0313-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-013-0313-2