Abstract
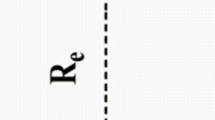
Structure, frequencies, H–H stretching frequency shifts, interaction energy, depth of the potential well and dissociation energy of the light cation–dihydrogen (M+–H2, where M = Li, Na, B, and Al) van der Waals complexes have been studied in detail using dispersion corrected double-hybrid and gradient-corrected density functional methods in conjunction with correlation consistent valence triple-ζ basis set. Equilibrium bond distance and dissociation energy agree very well with the experimental and theoretical values wherever available. The dissociation energies of Li+–H2, B+–H2, Na+–H2, and Al+–H2 van der Waals complexes calculated from the potential energy curves at mPW2PLYP-D/cc-pVTZ level are 4.83, 3.68, 2.42, and 1.25 kcal/mol, respectively, at a distances of 1.95, 2.25, 2.40, and 2.95 Å. Among all these complexes, Al+–H2 complex is comparatively less stable, as their dissociation energy as well as depth of the potential well are smaller compared to others complexes. The symmetry-adapted perturbation theory (SAPT) has been applied to quantify the nature of interactions. The SAPT results show that the contribution of dispersion and induction are significant, although electrostatic dominates.








Similar content being viewed by others
References
Burley SK, Petsko GA (1985) Science 229:23–28
Saenger W (1984) Principles of nucleic acid structure. Springer, New York
Lehn JM (1995) Supramolecular chemistry: concepts and perspectives. VCH, New York
Steed JW, Atwood JL (2000) Supramolecular chemistry: a concise introduction. Wiley, New York
Sygula A, Fronczek FR, Sygula R, Rabideau PW, Olmstead MM (2007) J Am Chem Soc 129:3842–3843
Sanz C, Bodo E, Gianturco FA (2005) Chem Phys 314:135–142
Emmeluth C, Poad BLJ, Thompson CD, Weddle GH, Bieske EJ (2007) J Chem Phys 126: 204309-1-204309-9
De Silva N, Njegic B, Gordon MS (2011) J Phys Chem A 115:3272–3278
Kraemer WP, Spirko V (2006) Chem Phys 330:190–203
Dryza V, Poad BLJ, Bieske EJ (2008) J Am Chem Soc 130:12986–12991
Kemper PR, Bushnell JE, Weis P, Bowers MT (1998) J Am Chem Soc 120:7577–7584
Poad BLJ, Wearne PJ, Bieske EJ, Buchachenko AA, Bennett DIG, Klos J, Alexander MH (2008) J Chem Phys 129:184306-1-184306-8
Emmeluth C, Poad BLJ, Thompson CD, Weddle G, Bieske EJ, Buchachenko AA, Grinev TA, Klos J (2007) J Chem Phys 127:164310-1-164310-8
Bushnell JE, Kemper PR, Bowers MT (1994) J Phys Chem 98:2044
Kemper PR, Bushnell J, Bowers MT, Gellene GI (1998) J Phys Chem A 102:8590–8597
Grimme S (2004) J Comput Chem 25:1463–1473
Grimme S (2006) J Comput Chem 27:1787–1799
Elstner M, Hobza P, Frauenheim T, Suhai S, Kaxiras E (2001) J Chem Phys 114:5149–5155
Wu X, Vargas MC, Nayak S, Lotrich V, Scoles G (2001) J Chem Phys 115:8748–8757
Parac M, Etinski M, Peric M, Grimme S (2005) J Chem Theory Comput 1:1110–1118
Thanthiriwatte KS, Hohenstein EG, Burns LA, Sherrill CD (2011) J Chem Theory Comput 7:88–96
Zhao Y, Lynch BJ, Truhlar DG (2004) J Phys Chem A 108:4786–4791
Grimme S (2006) J Chem Phys 124:034108-1-034108-15
Schwabe T, Grimme S (2006) Phys Chem Chem Phys 8:4398–4401
Schwabe T, Grimme S (2007) Phys Chem Chem Phys 9:3397–3406
Perdew JP, Burke K, Ernzerhof M (1996) Phys Rev Lett 77:3865–3868
Adamo C, Barone V (1998) J Chem Phys 108:664
Merecero JM, Maxtrain JM, Lopez X, York DM, Largo A, Eriksson LA, Ugalde JM (2005) Int J Mass Spectrum 240:37 and references therein
Zimmerli U, Parrinello M, Koumoutsakos P (2004) J Chem Phys 120:2693–2699
Grimme S, Antony J, Ehrlich S, Krieg H (2010) J Chem Phys 132:154104
Dunning TH (1989) J Chem Phys 90:1007–1023
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968
Neese F (2010) ORCA—an ab initio, density functional and semiempirical program package, Version 2.80, Institut fuer Physikalische und Theoretische Chemie, Universitaet Bonn, Germany
Zhurko GA, Zhurko DA (2010) Chemcraft, Vers. 1.6
Jeziorski B, Moszynski R, Szalewicz K (1994) Chem Rev 94:1887
Gordon MS, Schmidt MW (2005) Theory and applications of computational chemistry: the first forty years. Elsevier, Amsterdam
Schmidt MW, Baldridge KK, Boatz JA, Elbert ST, Gordon MS, Jensen JH, Koseki S, Matsunaga N, Nguyen KA, Su SJ, Windus TL, Dupuis M, Montgomery JA (1993) J Comput Chem 14:1347
Bukowski R, Cencek W, Jankowski P, Jeziorska M, Jeziorski B, Kucharski SA, Lotrich VF, Misquitta AJ, Moszynski R, Patkowski K et al (2009) SAPT2008: an ab initio program for many-body symmetry-adapted perturbation theory calculations of intermolecular interaction energies, sequential and parallel versions. University of Delaware and University of Warsaw, Newark
Jennings DE, Brault JW (1983) J Mol Spectrosc 102:265–272
Acknowledgments
Srimanta Pakhira thanks Amit Chakraborty, Department of Theoretical Physics (IACS), for his assistance. Kaushik Sen and Chandan Sahu are grateful to the Council of Scientific and Industrial Research (CSIR), Government of India, for providing research fellowships. We thank Professor Prasanta Kumar Mukherjee, Ramkrishna Mission Vivekananda University, Belur Math, for his valuable suggestions. Thanks are also due to the reviewer for his constructive comments and suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pakhira, S., Sahu, C., Sen, K. et al. Dispersion corrected double high-hybrid and gradient-corrected density functional theory study of light cation–dihydrogen (M+–H2, where M = Li, Na, B and Al) van der Waals complexes. Struct Chem 24, 549–558 (2013). https://doi.org/10.1007/s11224-012-0107-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-012-0107-y