Abstract
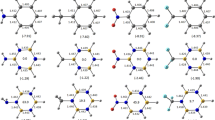
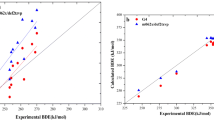
An extension of the harmonic oscillator model of aromaticity (HOMA) model to systems with carbon–boron bonds is presented. Model parameters were estimated using experimental and theoretical bond lengths. It is shown that both approaches produce very similar HOMA models. In the second part of the article, the aromaticity levels of several model compounds containing carbon–boron bonds are calculated using the previously obtained parameters. The results of these calculations are compared with those provided by other aromaticity indices. The aromaticity of boron-containing compounds is also compared with the aromaticity of analogous compounds containing carbon and nitrogen.



Similar content being viewed by others
References
Krygowski TM, Cyranski MK (2001) Chem Rev 101:1385–1420
Kruszewski J, Krygowski TM (1972) Tetrahedron Lett 13:3839–3842
Krygowski TM (1993) J Chem Inf Comput Sci 33:70–78
Krygowski TM, Cyranski MK (1996) Tetrahedron 52:1713–1722
Raczynska ED (2005) Pol J Chem 79:749–758
Zborowski KK, Proniewicz LM (2008) J Phys Org Chem 21:207–214
Zborowski KK, Proniewicz LM (2009) Pol J Chem 83:477–484
Madura ID, Krygowski TM, Cyranski MK (1998) Tetrahedron 54:14913–14918
Krogh-Jespersen K, Cremer D, Dill JD, Pople JA, PvR Schleyer (1981) J Am Chem Soc 103:2589–2594
Aihara J, Kanno H, Ishida T (2005) J Am Chem Soc 127:13324–13330
Del Bene JE, Yañez M, Alkorta I, Elguero J (2009) J Chem Theory Comp 5:2239–2247
Yu HL, Sang RL, Wu YY (2009) J Phys Chem A 113:3382–3386
Kiran B, Gopa Kumar G, Nguyen MT, Kandalam AK, Jena P (2009) Inorg Chem 48:9965–9967
Ruman T, Jarmuła A, Rode W (2010) Bioorg Chem 38:242–245
Pauling L (1947) J Am Chem Soc 69:542–553
Krygowski TM, Cyranski MK (1996) Tetrahedron 52:10255–10264
Schleyer PvR, Marker C, Dransfeld A, Jiao HJ, Hommes NJRV (1996) J Am Chem Soc 118:6317–6318
Schleyer PvR, Manoharan M, Wang ZX, Kiran B, Jiao HJ, Puchta R, Hommes NJRV (2001) Org Lett 3:2465–2468
Corminboeuf C, Heine T, Seifert G, Schleyer PvR, Weber J (2004) Phys Chem Chem Phys 6:273–276
Sola M, Feixas F, Jimenez-Halla JOC, Matito E, Poater J (2010) Symmetry 2:1156–1179
Palusiak M, Krygowski TM (2007) Chem Eur J 13:7996–8006
Bader RFW (1990) Atoms in molecules. A quantum theory. Oxford University, New York
Keith TA, AIMAll (Version 09.11.08, standard), TK Gristmill Software, Overland Park KS, USA, 2009. http://aim.tkgristmill.com
Møller C, Plesset MS (1934) Phys Rev 46:618–622
Woon DE, Dunning TH Jr (1993) J Chem Phys 98:1358–1372
Becke AD (1993) J Chem Phys 98:5648–5653
Krishnan R, Binkley JS, Seeger R, Pople JA (1980) J Chem Phys 72:650–655
Wolinski K, Hilton JF, Pulay P (1990) J Am Chem Soc 112:8251–8260
Gaussian 03, Revision C.02, Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross J, Bakken BV, Adamo C, Jaramillo J, Gomperts JR, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PA, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian, Inc., Wallingford, CT
Boese R, Paetzold P, Tapper A, Ziembinski R (1989) Chem Ber 122:1057–1060
Schleyer PvR, Jiao H, van Eikema Hommes JR, Malkin VG, Malkina OL (1997) J Am Chem Soc 119:12669–12670
Acknowledgments
The authors are grateful to Prof. Tadeusz M. Krygowski for advice and helpful discussions. We also thank the Academic Computer Centre “Cyfronet” Krakow for funding the computer time. We thank the Ministerio de Ciencia e Innovación (Project No. CTQ2009-13129-C02-02) and the Comunidad Autónoma de Madrid (Project MADRISOLAR2, ref. S2009/PPQ-1533) for continued support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zborowski, K.K., Alkorta, I., Elguero, J. et al. Calculation of the HOMA model parameters for the carbon–boron bond. Struct Chem 23, 595–600 (2012). https://doi.org/10.1007/s11224-011-9907-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9907-8