Abstract
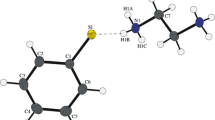
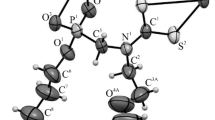
A series of N,N’-disubstituted 3,4-ethylenedioxythiophene-2,5-dicarboxamides was synthesised by amide bond formation between 3,4-ethylenedioxythiophene-2,5-dicarbonyl chloride and corresponding primary amines, where the size and the nature of the substituent were varied. The crystal structures of prepared compounds were determined by X-ray structure analysis. Mechanism and reaction rates of interconversion between conformational isomers were obtained by DFT calculations. All studied compounds reveal axial chirality with molecular symmetry C 2. Amide bond isomerisation and twisting of the dioxane ring in studied compounds results in the formation of series of conformers of which the s-trans/s-trans conformer is energetically most favourable.








Similar content being viewed by others
References
Yang J, Kim DH, Hendricks JL, Leach M, Northey R, Martin DC (2005) Acta Biomater 1:125–136
Bello A, Giannetto M, Mori G, Seeber R, Terzi F, Zanardi C (2007) Sens Actuators B 121:430–435
Roncali J, Blanchard P, Frère P (2005) J Mater Chem 15:1589–1610
Liu R, Cho SI, Lee SB (2008) Nanotechnology 19:215710–215718
Stolić I, Mišković K, Magdaleno A, Silber AM, Piantanida I, Bajić M, Glavaš-Obrovac Lj (2009) Bioorg Med Chem 17:2544–2554
Stolić I, Mišković K, Piantanida I, Baus-Lončar M, Glavaš-Obrovac Lj, Bajić M (2011) Eur J Med Chem 46:743–755
Rahman MA, Kumar P, Park DS, Shim YB (2008) Sensors 8:118–141
Rao SS, Winter JO (2009) Front Neuroeng 2:1–14
Bally M, Voros J (2009) Nanomedicine 4:447–467
Kros A, Nolte RJM, Sommerdijk NAJM (2002) J Polym Sci A 40:738–747
Luo SC, Ali EM, Tansil NC, Yu HH, Gao S, Kantchev EAB, Ying JY (2008) Langmuir 24:8071–8077
Groenendaal L, Jonas F, Freitag D, Pielartzik H, Reynolds JR (2000) Adv Mater 12:481–494
Laughton CA, Tanious F, Nunn CM, Boykin DW, Wilson WD, Neidle S (1996) Biochemistry 35:5655–5661
Hall JE, Kerrigan JE, Ramachandran K, Bender BC, Stanko JP, Jones SK, Patric DA, Tidwell RR (1998) Antimicrob Agents Chemother 42:666–674
Sotzing GA, Reynolds JR, Steel PJ (1996) Chem Mater 8:882–889
Abboud KA, Irvin DJ, Reynolds JR (1998) Acta Crystallogr C 54:1994–1997
Kumar A, Welsh DM, Morvant MC, Piroux F, Abboud KA, Reynolds JR (1998) Chem Mater 10:896–902
Ono K, Tomura M, Saito K (2008) Acta Crystallogr E 64:o468
Djukic B, Harrington LE, Britten JF, Lemaire MT (2008) Acta Crystallogr E 64:o463
Molčanov K, Stolić I, Kovačević G, Kojić-Prodić B, Bajić M (2011) J Mol Struct 987:174–179
Dodziuk H, Mirowicz M (1990) Tetrahedron Asymmetry 1:171–186
Dodziuk H (1992) Tetrahedron Asymmetry 3:43–50
CrysAlis PRO (2007) Oxford Diffraction Ltd, UK
Sheldrick GM (2008) Acta Crystallogr A64:112–122
Spek AL (2003) J Appl Crystallogr 36:7–13
Farrugia LJ (1997) J Appl Crystallogr 30:565
Macrae CF, Edgington PR, McCabe P, Pidcock E, Shields GP, Taylor R, Towler M, Van De Streek J (2006) J Appl Crystallogr 39:453–457
Zhao Y, Thrular DG (2008) Theor Chem Acc 120:215–241
Coffey M, McKellar BR, Reinhardt BA, Nijakowski T, Feld WA (1996) Synth Commun 26:2205–2212
Cremer D, Pople JA (1975) J Am Chem Soc 97:1354–1358
Vereshchagin AN (1983) Russ Chem Rev 52:1081–1095
Durig JR, Carter RO, Carreira LA (1974) J Chem Phys 60:3098–3104
Choo J, Yoo S, Moon S, Kwon Y, Chung H (1998) Vib Spectrosc 17:173–182
Tecklenburg MM, Looney J (1989) J Am Chem Soc 111:6920–6926
Acknowledgments
The financial support from the Croatian Ministry of Science, Education and Sports (Programs 098-1191344-2943 and 053-0982914-2965) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Stolić, I., Molčanov, K., Kovačević, G. et al. Axial chirality of N,N′-disubstituted 3,4-ethylenedioxythiophene-2,5-dicarboxamides. Struct Chem 23, 425–432 (2012). https://doi.org/10.1007/s11224-011-9885-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9885-x