Abstract
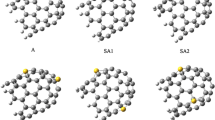
The structures of mono- and di-podal pyrrolic amides functionalized (5,5) single-walled carbon nanotubes (SWCNTs) and their complexes with fluoride, chloride, and bromide ions were obtained using the two-layered ONIOM(MO:MO) and density functional theory (DFT) methods. The binding energies between halide ions and all the receptors and their charge transfers were obtained using DFT method. The computational results indicate that the pyrrolic amide functionalized on the SWCNT affects to the density of state and energy gap of SWCNT. All the free receptors, mono-, di-podal pyrrolic amides and the functionalized SWCNT forming the strongest complexes were found.







Similar content being viewed by others
References
Iijima S, Ichihashi T (1993) Nature 363:603
Singh P, Campidelli S, Giordani S, Bonifazi D, Bianco A, Prato M (2009) Chem Soc Rev 38:2214
Michael J (2006) Carbon nanotubes: properties and applications. CRC Press, Taylor and Francis Group, LLC, Boca Raton
Schluter AD (2005) Functional molecular nanostructures. Springer, New York
Yang W, Thordarson P, Gooding JJ, Ringer SP, Braet F (2007) Nanotechnology 18(41):412001
Bianco A, Kostarelos K, Partidos CD, Prato M (2005) Chem Commun 5:571
Chu H, Wei L, Cui R, Wang J, Li Y (2010) Coord Chem Rev 254:1117
Mercuri F, Sgamellotti A (2007) Inorg Chim Acta 360:785
Steed JW, Atwood JL (2009) Supramolecular chemistry. Wiley, England
Gale PA (2011) Chem Comm 47:82
Sessler JL, Gelloz GB, Gale PA, Camiolo S, Anslyn EV, Anzenbacher P Jr, Furuta H, Kirkovits GJ, Lynch VM, Maeda H, Morosini P, Scherer M, Shriver J, Zimmerman RS (2003) Polyhedron 22:2963
Sessler JL, Camiolo S, Gale PA (2003) Coord Chem Rev 240:17
Bondy CR, Loeb SJ (2003) Coord Chem Rev 240:77
Zhang Z, Schreiner PR (2009) Chem Soc Rev 38:1187
Yin Z, Li Z, Yu A, He J, Cheng JP (2004) Tetrahedron Lett 45:6803
Zhenming Y, Shangyuan L (2009) Chin J Chem 27:43
Chidawanyika W, Nyokong W (2010) Carbon 48:2831
Holzinger M, Abraham J, Whelan P, Graupner R, Ley L, Hennrich F, Kappes M, Hirsch A (2003) J Am Chem Soc 125:8566
Becke AD (1988) Phys Rev A 38:3098
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:385
Maseras F, Morokuma K (1995) J Comput Chem 16:1170
Humbel S, Sieber S, Morokuma K (1996) J Chem Phys 105:1959
Stewart JJP (1989) J Comput Chem 10:209
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2008) Gaussian 03, revision E.01. Gaussian, Inc, Wallingford
Flükiger P, Lüthi HP, Portmann S, Weber J (2000) Molekel 4.3. Swiss Center for Scientific Computing, Manno
O’Boyle NM, Tenderholt AL, Langner KM (2008) J Comput Chem 29:839
Acknowledgments
The authors would like to appreciate the Research Affairs, Kosumwittayasan School, Maha Sarakham Province, for partial support of this research and the facility provided by Supramolecular Chemistry Research Unit, Department of Chemistry, Faculty of Science, Mahasarakham University. The Institute for the Promotion of Teaching Science and Technology, Thailand, for financial support is also gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tetasang, S., Keawwangchai, S., Wanno, B. et al. Quantum chemical investigation on structures of pyrrolic amides functionalized (5,5) single-walled carbon nanotube and their binding with halide ions. Struct Chem 23, 7–15 (2012). https://doi.org/10.1007/s11224-011-9839-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9839-3