Abstract
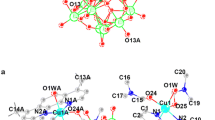
A proton-conductive supramolecular complex, {[Cu(H2O)8][H(H2O)3](HINO)4(PMo12O40)} n , was constructed by a self-assembly of H+(H2O)3 clusters, [Cu(H2O)8]2+ clusters, [PMo12O40]3− anions, and isonicotinic acid N-oxide (HINO). Single-crystal X-ray diffraction analysis at 293 K revealed that the complex presented the three-dimensional (3D) supramolecular framework built from non-covalent interactions. Interestingly, [PMo12O40]3− anions self-assembled into poly-Keggin-anion chains in the supramolecular framework. Thermogravimetric analysis shows no weight loss in the temperature range of 20–100 °C, indicating that all water molecules in the unit structure are not easily lost below 100 °C. Surprisingly, the proton conductivity of the complex in the temperature range of 85–100 °C under 98% RH condition reached good proton conductivity of 10−3 S cm−1. A possible mechanism of the proton conduction was proposed according to the experimental results.
Graphical Abstract
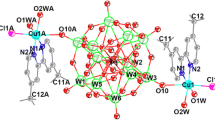
A good proton-conductive supramolecular complex was built from non-covalent interactions among HINO molecules, [Cu(H2O)8]2+ and H+(H2O)3 clusters, and [PMo12O40]3− anions, as well as its structure was determined by using single-crystal X-ray diffraction data.









Similar content being viewed by others
References
Wood BC, Marzari N (2007) Phys Rev B 76:134301
Yu R, Jonghe LCD (2007) J Phys Chem C 111:11003
Kreuer KD, Paddison SJ, Spohr E, Schuster M (2004) Chem Rev 104:4637
Nakamura O, Kodama T, Ogino I, Miyake Y (1979) Chem Lett 17
Casciola M, Constantino U (1986) Solid State Ionics 20:69
Misono M (2001) Chem Commun 1141
Slade RCT, Barker J, Pressman HA, Strange JH (1988) Solid State Ionics 28–30:594
Katsoulis DE (1998) Chem Rev 98:359
Malers JL, Sweikart MA, Horan JL, Turner JA, Herring AH (2007) J Power Sources 172:83
Herring AM (2006) Polym Rev 46:245
Alberti G, Casciola M, Costantino U, Peraio A, Rega T (1995) J Mater Chem 5:1809
Alberti G, Casciola M (2001) Solid State Ionics 145:3
Sang XG, Wu QY, Pang WQ (2003) Mater Chem Phys 82:405
Honma I, Nomura S, Nakajima H (2001) J Memb Sci 185:83
Kim JD, Honma I (2005) Solid State Ionics 176:547
Kim YS, Wang F, Hickner M, Zawodzinski TA, McGrath JE (2003) J Memb Sci 212:263
Tazi B, Savadogo O (2000) Electrochim Acta 45:4329
Kozhevnikov IV (2007) J Mol Catal A Chem 262:86
Ramani V, Kunz HR, Fenton JM (2004) J Memb Sci 232:31
Inzelt G, Pineri M, Schultze JW, Vorotyntsev MA (2000) Electrochim Acta 45:2403
Verma A, Scott K (2010) J Solid State Electrochem 14:213
Li MQ, Shao ZG, Scott K (2008) J Power Sources 183:69
Claude RD, Michel F, Raymonde F, Rene T (1983) Inorg Chem 22:207
Simapson PG, Vinciguerra A, Quagliano JV (1963) Inorg Chem 2:282
Wu QY, Zhao SL, Wang JM, Zhang JQ (2007) J Solid State Electrochem 11:240
SMART and SAINT (1996) Area detector control and integration software. Siemens Analytical X-ray Systems, Inc., Madison, WI
Sheldrick GM (1997) SHELXTL V5.1, software reference manual. Bruker AXS, Inc., Madison, WI
Wei ML, He C, Hua WJ, Duan CY, Li SH, Meng QJ (2006) J Am Chem Soc 128:13318
Wei ML, He C, Sun QZ, Meng QJ, Duan CY (2007) Inorg Chem 46:5957
Duan CY, Wei ML, Guo D, He C, Meng QJ (2010) J Am Chem Soc 132:3321
Wei ML, Xu HY, Sun RP (2009) J Coord Chem 62:1989
Wei ML, Zhuang PF, Li HH, Yang YH (2011) Eur J Inorg Chem 1473
Alizadeh MH, Eshtiagh-Hosseini H, Mirzaei M, Salimi AR, Razavi H (2008) Struct Chem 19:155–164
Sadakiyo M, Yamada T, Kitagawa H (2009) J Am Chem Soc 131:9906
England WA, Cross MG, Hamnett A, Wiseman PJ, Goodenough JB (1980) Solid State Ionics 1:231–249
Kanda Y, Lee KY, Nakata S, Asaoka S, Misono M (1988) Chem Lett 139
Yang J, Janik MJ, Ma D, Zheng A, Zhang M, Neurock M, Davis RJ, Ye C, Deng F (2005) J Am Chem Soc 127:18274
Slade RCT, Hardwick A, Dickens PG (1983) Solid State Ionics 9–10:1093
Supplit R, Sugawara A, Peterlik H, Kikuchi R, Okubo T (2010) Eur J Inorg Chem 3993
Janic MJ, Davis RJ, Neurock M (2005) J Am Chem Soc 127:5238
Hayashi EG, Moffat JB (1983) Catal J 83:192
Kreuer KD, Rabenau A, Weppner W (1982) Angew Chem Int Ed 21:208
Agmon N (1995) Chem Phys Lett 244:456
Acknowledgments
This work was supported by the National Natural Science Foundation of China (no. 20971038), the Natural Science Foundation of the Education Department of Henan Province (no. 2009A150015), and the Science Foundation for Youths of Henan Normal University (2008qk10).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wei, ML., Li, HH., Wang, XX. et al. A supramolecular complex based on poly-Keggin-anion chains: synthesis, structure characterization, and conductivity. Struct Chem 23, 129–136 (2012). https://doi.org/10.1007/s11224-011-9790-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-011-9790-3