Abstract
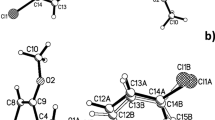
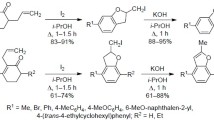
A series of benzyloxybenzaldehyde derivatives (1–3) were prepared by the reactions of 4-nitrobenzyl bromide with 4-hydroxy-3-methoxybenzaldehyde (vanillin), 2-hydroxy-3-methoxybenzaldehyde (o-vanillin) and 2-hydroxy-4-methoxybenzaldehyde. When the reaction time is quite long, benzofuran derivatives (4 and 5) were obtained by the reactions of ortho-hydroxyaldehydes with the 4-nitrobenzyl bromide. Condensation reactions among the three benzyloxybenzaldehyde derivatives (1–3) with 2-aminomethylfuran (furfurylamine) yielded the new imine compounds (6–8). The structures of these aldehydes (1–3), benzofuran derivatives (4 and 5) and imine compounds (6–8) were confirmed on the basis of elemental analyses, IR, 1H NMR and 13C NMR and mass spectroscopy. The solid-state structures of compounds 4–6 were determined by single-crystal X-ray crystallography.





Similar content being viewed by others
References
Rohini RM, Kumar DRH, Kumar RR, Glaate M (2008) J Pharm Res 7:239–242
Nishat N, Parveen S, Dhyani S (2009) J Coord Chem 62:1091–1099
Garoufis A, Hadjikakou SK, Hadjiliadis N (2009) Coord Chem Rev 253:1384–1397
Anbu S, Kandaswamy M, Suthakaran P, Murugan V, Varghese B (2009) J Inorg Biochem 103:401–410
Bagihalli GB, Avaji PG, Patil SA, Badami PS (2008) Eur J Med Chem 43:2639–2649
Hayvali Z, Gündüz N, Kiliç Z, Weber E (2000) Z Naturforsch 55b:975–981
Hayvalı Z, Gündüz N, Kiliç Z, Weber E (1999) J Prakt Chem 341:568–573
Hökelek T, Gündüz N, Hayvali Z, Kiliç Z (1995) Acta Cryst C51:880–884
Campbell EJ, Nguyen ST (2000) Tetrahedron Lett 42:1221–1225
Higasio Ya S, Shoji T (2001) Appl Catal A Gen 221:197–207
Juhasz L, Dinya Z, Antus S, Gunda TE (2000) Tetrahedron Lett 41:2491–2494
Marion F, Williams DE, Patrick BO, Hollander I, Mallon R, Kim SC, Roll DM, Feldberg L, Van Soest R, Andersen RJ (2006) J Org Lett 8:321–324
Boukouvalas J, Pouliot M, Robichaud J, MacNeil S, Snieckus V (2006) J Org Lett 8:3597–3599
Lipshutz Bruce H (1986) Chem Rev 86:795–819
Chen C-Y, Dormer PG (2005) J Org Chem 70:6964–6967
Zhou G, Corey EJ (2005) J Am Chem Soc 127:11958–11959
Ono M, Kawashima H, Nonaka A, Kawai T, Haratake M, Mori H, Kung M-P, Kung HF, Saji H, Nakayama M (2006) J Med Chem 49:2725–2730
Baker SR, Cases M, Keenan M, Lewis RA, Tan P (2003) Tetrahedron Lett 44:2995–2999
Serra S, Fuganti C (2003) Synlett 2005–2008
Dahlen A, Petersson A, Hilmersson G (2003) Org Biomol Chem 1:2423–2426
Horaguchi T, Iwanami H, Tanaka T, Hasegawa E, Shimizu T (1991) J Chem Soc Chem Commun 44–46
Horaguchi T, Kobayashi H, Miyazawa K, Hasegawa E, Shimizu T, Suzuki T, Tanemura K (1990) J Heterocycl Chem 27:935–940
Kanazawa C, Goto K, Terada M (2009) Chem Commun 5248–5250
Wang Z, Gu J, Jing H, Liang Y (2009) Synth Commun 39:4079–4087
Sahm W, Schinzel E, Juerges P (1974) Liebigs Ann der Chemie 4:523–538
Bruker (2005) SADABS Bruker AXS Inc., Madison, WI, USA
Sheldrick GM (2008) Acta Cryst A 64:112–122
Petchmanee T, Ploypradith P, Ruchirawat S (2006) J Org Chem 71:2892–2895
Plourde GL, Spaetzel RR (2002) Molecules 7:697–705
Li M, Chen X (2008) Acta Cryst E 64:o2291
Kraus GA, Zhang N, Verkade JG, Nagarajan M, Kisanga PB (2000) Org Lett 2:2409–2410
Farrugia LJ (1997) J Appl Cryst 30:565
Spek AL (2003) J Appl Cryst 36:7–13
Acknowledgements
The authors gratefully acknowledge the financial assistance of the Scientific and Technical Research Council of Turkey (TUBITAK), Grand No. TBAG 109T034. We also thank Anadolu University and Medicinal Plants and Medicine Research Center of Anadolu University Eskişehir, for allowing us to use the X-ray facility.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hayvali, Z., Dal, H., Köksal, P. et al. Syntheses, spectroscopic and crystallographic characterizations of 4-nitrobenzyloxy derivatives and benzofurans from ortho-substituted benzaldehydes and 4-nitrobenzyl bromide. Struct Chem 21, 837–845 (2010). https://doi.org/10.1007/s11224-010-9618-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9618-6