Abstract
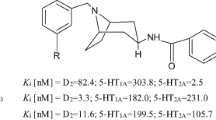
Schizophrenia is a debilitating mental disorder which affects approximately 1% of the world’s population. Clozapine is an atypical antipsychotic showing unmatched effectiveness in the control of treatment-resistant schizophrenia. Unlike typical antipsychotics, clozapine does not induce extrapyramidal side effects (EPS), tardive dyskinesia or elevate prolactin levels. However, clozapine can induce a potentially fatal blood disorder, agranulocytosis, in 1–2% of patients, severely limiting its clinical use. The model for antipsychotic activity under investigation is based on obtaining a clozapine-like profile with preferential dopamine D4 and serotonin 5-HT2A receptor affinity. Profiled herein are three unique members of a series of prospective antipsychotic agents. Compound (I) originated from the structural hybridization of the commercial therapeutics, clozapine and haloperidol, whilst compounds (II) and (III) possess an alternative tricyclic nucleus derived from JL13; a clozapine-like atypical antipsychotic developed by Liégeois et al. These compounds have been synthesized and characterized by means of elemental analysis, IR, 1H and 13C-NMR spectroscopy, MS and X-ray diffraction. Compound (I) crystallizes in space group P(−1) with a = 10.5032(1), b = 10.6261(2), c = 12.6214(3) Å, α = 81.432(1)°, β = 83.292(1)°, γ = 61.604(1)°, Z = 2, V = 1223.62(4) Å3, C28H29ClN4O, M r = 473.00, D c = 1.284 Mg/m3, μ = 0.185 mm−1, F(000) = 500, R = 0.0506 and wR = 0.1304. Compound (II) crystallizes in the monoclinic space group P21/c with a = 10.8212(2), b = 9.3592(2), c = 22.9494(5) Å, β = 106.471(1)°, Z = 4, V = 2228.88(8) Å3, C25H25ClN4O2, M r = 448.94, D c = 1.338 Mg/m3, μ = 0.202 mm−1, F(000) = 944, R = 0.0529 and wR = 0.1129. Compound (III) crystallizes in the monoclinic space group P21/c with a = 10.5174(2), b = 9.3112(2), c = 24.2949(5) Å, β = 98.666(1)°, Z = 4, V = 2352.03(8) Å3, C25H24Cl2N4O2, M r = 483.38, D c = 1.365 Mg/m3, μ = 0.306 mm−1, F(000) = 1008, R = 0.0478 and wR = 0.1067. The solid state conformations of (I), (II) and (III) exhibit the characteristic V-shaped buckled nature of the respective dibenzodiazepine and pyridobenzoxazepine nuclei with the central seven-membered heterocycle in a boat conformation. The molecules of (I) form a head-to-tail dimeric motif stabilized by hydrogen bonding. The results of a conformational analysis of compounds (I)–(III) investigating the effect of environment (in vacuo and aqueous solution) are presented. These analogues were tested for in vitro affinity for the dopamine D4 and serotonin 5-HT2A receptors and their comparative receptor binding profiles to clozapine and JL13 are reported.












Similar content being viewed by others
References
Jablensky A, Sartorius N, Korten A, Ernberg G, Anker M, Cooper JE, Day JR (1987) Br J Psychiatr 151:408
Andreasen NC (2000) Brain Res Brain Res Rev 31:106
Capuano B, Crosby IT, Lloyd EJ (2002) Curr Med Chem 9:521
Andreasen NC (1995) Lancet 346:477
De Oliveira IR, Juruena MF (2006) J Clin Pharm Ther 31:523
Alves Fda S, Figee M, Vamelsvoort T, Veltman D, Haan L (2008) Psychopharmacol Bull 41:121
Toda M, Abi-Dargham A (2007) Curr Psychiatr Rep 9:329
Carlsson A (1977) Psychol Med 7:583
Creese I, Burt DR, Snyder SH (1976) Science 192:481
McKenna PJ (1987) Br J Psychiatry 151:288
Meltzer HY, Stahl SM (1976) Schizophr Bull 2:19
Seeman P, Lee T, Chau-Wong M, Wong K (1976) Nature 261:717
Snyder SH (1976) Am J Psychiatry 133:197
Wooley DW, Shaw EN (1957) Ann NY Acad Sci 66:649
Harrison PJ, Geddes JR (1996) Lancet 347:1274
Harrison PJ, Burnet PW (1997) J Psychopharmacol 11:18
Akhondzadeh S (2001) IDrugs 4:295
Hurlemann R, Boy C, Meyer PT, Scherk H, Wagner M, Herzog H, Coenen HH, Vogeley K, Falkai P, Zilles K, Maier W, Bauer A (2005) Anat Embryol 210:519
De Oliveira IR, Juruena MFJ (2006) Clin Pharm Ther 31:523
Tamminga CA (2006) Am J Psychiatry 163:563
Uetrecht JP (1992) Drug Saf 7:S51
Liu ZC, Uetrecht JP (1995) J Pharmacol Exp Ther 275:1476
Pirmohamed M, Park K (1997) CNS Drugs 7:139
Capuano B, Crosby IT, McRobb FM, Podloucka A, Taylor DA, Vom A, Yuriev E (2010) Aust J Chem 63:116
Capuano B, Crosby IT, Lloyd EJ, Podloucka A, Taylor DA (2008) Aust J Chem 61:930
Capuano B, Crosby IT, Lloyd EJ, Podloucka A, Taylor DA (2003) Aust J Chem 56:875
Capuano B, Crosby IT, Lloyd EJ, Taylor DA (2002) Aust J Chem 55:565
Lima LM, Barreiro EJ (2005) Curr Med Chem 12:23
Liégeois JF, Rogister FA, Bruhwyler J, Damas J, Nguyen TP, Inarejos M-O, Chleide EMG, Mercier MGA, Delarge JE (1994) J Med Chem 37:519
Mouithys-Mickalad A, Kauffmann JM, Petit C, Bruhwyler J, Liao Y, Wikstrom H, Damas J, Delarge J, Deby-Dupont G, Geczy J, Liégeois JF (2001) J Med Chem 44:769
Liégeois JF, Zahid N, Bruhwyler J, Uetrecht J (2000) Arch Pharm Pharm Med Chem 333:63
Liégeois JF, Mouithys-Mickalad A, Bruhwyler J, Delarge J, Petit C, Kauffmann JM, Lamy M (1997) Biochem Biophys Res Commun 238:252
Gottlieb HE, Kotlyar V, Nudelman A (1997) J Org Chem 62:7512
Still C, Kahn M, Mitra A (1978) J Org Chem 43:2923
Blessing RH (1997) J Appl Cryst 30:421
Sheldrick GM (1997) SHELX97, program system for the solution and refinement of crystal structures. Universität Göttingen, Germany
Seeman P, Van Tol HHM (1994) Trends Pharmacol Sci 15:264
Allen FH (2002) Acta Crystallogr B58:380
Bruno IJ, Cole JC, Kessler M, Luo J, Motherwell WDS, Purkis LH, Smith BR, Taylor R, Cooper RI, Harris SE, Orpen AG (2004) J Chem Inf Comput Sci 44:2133
Petcher TJ, Weber H-P (1976) J Chem Soc Perkin Trans 2:1415
Dupont L, Liégeois JF (2003) Acta Crystallogr E59:o1962
Capuano B, Crosby IT, Forsyth CM, Lloyd EJ, Vom A, Yuriev E (2008) Acta Crystallogr E64:o1865
Miller DD, Harrold M, Wallace RA, Wallace LJ, Uretsky NJ (1988) Trends Pharmacol Sci 9:282
Hjerde E, Dahl SG, Sylte I (2005) Eur J Med Chem 40:185
Yuriev E, Kong DCM, Iskander MN (2004) Eur J Med Chem 39:835
Schwartz JC, Carlsson M, Caron M, Scatton B, Civelli O, Kebabian JW, Langer SZ, Sedvall G, Seeman P, Spano PF, Sokoloff P, Van Tol HHM (1998) Dopamine Receptors; The IUPHAR compendium of receptor characterization and classification, 1st edn. IUPHAR Media, London, p 141
Humphrey PP, Hartig P, Hoyer D (1993) Trends Pharmacol Sci 14:233
Martin GR (1998) 5-Hydroxytryptamine receptors; The IUPHAR compendium of receptor characterization and classification, 1st edn. IUPHAR Media, London, p 167
Fillers JP, Hawkinson SW (1982) Acta Crystallogr B38:1750
Capuano B, Crosby IT, Gable RW, Lloyd EJ (2000) Acta Crystallogr C56:339
Capuano B, Crosby IT, Egan SJ, Fallon GD, Lloyd EJ, Neve JE (2005) Acta Crystallogr E61:o20
Cosulich DB, Lovell FM (1977) Acta Crystallogr B33:1147
Dupont L, Liegeois JF (2004) Z Krist 219:147
Dupont L, Dideberg O, Liegeois JF, Delarge J (1987) Acta Crystallogr C43:716
Capuano B, Crosby IT, Forsyth CM, Lloyd EJ, Vom A, Yuriev E (2006) Acta Crystallogr E62:o5434
Liao Y, Venhuis BJ, Rodenhuis N, Timmerman W, Wikstroem H, Meier E, Bartoszyk GD, Boettcher H, Seyfried CA, Sundell S (1999) J Med Chem 42:2235
Okamoto K, Fujii S, Tomita K (1993) Acta Crystallogr C49:1125
Estrada E, Gonzalez JC, Santana L, Uriarte E, Castineiras A (2000) Struct Chem 11:249
Acknowledgements
We gratefully acknowledge financial support from Monash University, Australia (VCP Small Grants Scheme #3652166).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Capuano, B., Crosby, I.T., Forsyth, C.M. et al. New hybrids of clozapine and haloperidol and their isosteric analogues: synthesis, X-ray crystallography, conformational analysis and preliminary pharmacological evaluation. Struct Chem 21, 613–628 (2010). https://doi.org/10.1007/s11224-010-9591-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-010-9591-0