Abstract
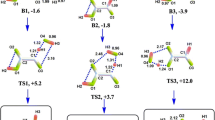
Gas phase reaction between germane GeH4 and water H2O was investigated at CCSD(T)/[aug-cc-pVTZ-pp for Ge + Lanl2dz for H and O]//MP2/6-31G(d,p) level. Only the hydrogen elimination channels are monitored. Within the energy range of 100 kcal/mol, we located nine equilibrium and six transition states on the potential energy surface (PES) of the Ge–O–H systems. GeH4 reacts with H2O exothermically (by 2.37 kcal/mol) without a barrier to form a non-planar complex GeH4·H2O which isomerizes to GeH3OH·H2 and H2GeOH2·H2 with a barrier of 44.34 kcal/mol and 53.75 kcal/mol respectively. The first step of hydrogen elimination gives two non-planar species, GeH3OH and H2GeOH2 but germinol GeH3OH is found to be more stable. Further thermal decomposition reactions of GeH3OH involving hydrogen elimination have been studied extensively using the same method. The final hydrogen elimination step gives HGeOH which can exist in cis and trans forms. As the trans form is more stable, only the trans form is considered on the potential energy surface (PES) of the reaction. The important thermochemical parameters (∆rEtot + ZPE), ∆rH and ∆rG for the H2 elimination pathways are predicted accurately.




Similar content being viewed by others
References
Meyerson B (2000) IBM J Res Develop 44:391
Kuhn K, Agostinelli M, Ahmed S, Chambers S, Cea S, Christensen S, Fischer P, Gong J, Kardas C, Letson T, Henning L, Murthy A, Muthali H, Obradovic B, Packan P, Pae SW, Post I, Putna S, Raol K, Roskowski A, Soman R, Thomas T, Vandervoorn P, Weiss M, Young I (2002) Proceeding of the IEDM, December 9, p 73
Ceiler MF, Kohl PA Jr, Bidstrup SA (1995) J Electrochem Soc 142:2067
Dominguez C, Rodriguez JA, Riera M, Llobera A, Diaz B (2003) J Appl Phys 93:5125
So SP (2001) J Phys Chem A 105:4988
Hu S-W, Wang Y, Wang X-Y, Chu T-W, Liu X-Q (2004) J Phys Chem A 108:1448
Ibuta N, Sagara F, Doi K, Nakamura K, Tachibana A, Ishihara Y, Suzuki K (2005) Jpn J Appl Phys 44:4133
Zhang Q, Zhang D, Wang S, Gu Y (2002) J Phys Chem A 106:122
Wang L, Zhang J (2004) J Phys Chem A 108:10346
Head-Gordon M, Pople JA, Frisch MJ (1988) Chem Phys Lett 153:503
Peterson KA (2003) J Chem Phys 119:11099
Pople JA, Head-Gordon M, Raghavachari K (1987) J Chem Phys 87:5968
Raghunath P, Lin MC (2007) J Phys Chem A 111:6481
Curtiss LA, Raghavachari K, Pople JA (1993) J Chem Phys 98:1293
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskortz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian 03, revision B.01. Gaussian Inc., Pittsburgh
Ignatov SK (2004) Moltran v.2.5—Program for molecular visualization and thermodynamic calculations, University of Nizhny Novgorod, http://ichem.unn.ru/Moltran. Accessed 5 April 2009
Acknowledgments
We thank Aneesur Rahman Centre for High Performance Computing of IACS for providing us the computational facility. B. M gratefully acknowledges the Council of Scientific and Industrial Research (CSIR), Government of India for a junior research fellowship. Thanks are due to the reviewer for his valuable comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mondal, B., Bhattacharyya, I., Ghosh, D. et al. Potential energy surface and thermochemistry for the direct gas phase reaction of germane and water. Struct Chem 20, 851–858 (2009). https://doi.org/10.1007/s11224-009-9483-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9483-3