Abstract
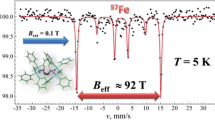
The compound Fe[C(SiMe3)3]2 has been prepared and investigated by the means of powder X-ray diffractometry and 57Fe Mössbauer spectroscopy. The compound’s unique geometry, in which iron is linearly coordinated by the two C(SiMe3)3 ligands, results in a unusual electronic structure of iron, which is visualized as an extreme high hyperfine magnetic field of 157.5(8) T as sensed by the 57Fe nucleus at T = 20 K. In order to obtain information on the electronic structure of iron and on the bonds to the ligands, DFT (density functional theory) calculations were carried out on Fe[C(SiMe3)3]2. The high-spin state of iron was found to be energetically favored: an Fe(II) electron configuration of 3d5.83 4s0.72 is predicted, where the 4s electron density is only slightly polarized, and most of the unpaired electrons have 3d character. By assuming a linear crystal field, and associated 3d level scheme as a starting point, it is suggested that the extreme high hyperfine magnetic field, observed along with an apparently negative quadrupole splitting, is perpendicular to the C–Fe(II)–C bond axis, and can be decomposed mainly into contact (B c ≈ 44 T), dipolar (B d ≈ 14 T), and orbital (B L ≈ 99 T) hyperfine magnetic field contributions.





Similar content being viewed by others
References
Reiff WM, Frommen CM, Yee GT, Sellers SP (2000) Inorg Chem 39:2076. doi:10.1021/ic990910l
Andres H, Bominaar EL, Smith JM, Eckert NA, Holland PL, Münck E (2002) J Am Chem Soc 124:3012. doi:10.1021/ja012327l
Reiff WM, LaPointe AM, Witten EH (2004) J Am Chem Soc 126:10206. doi:10.1021/ja030632w
Vértes A, Korecz L, Burger K (1979) Mössbauer spectroscopy. Akadémiai Kiadó, Budapest
LaPointe AM (2003) Inorg Chim Acta 345:359. doi:10.1016/S0020-1693(02)01309-9
Klencsár Z, Kuzmann E, Vértes A (1996) J Rad Nucl Chem 210:105. doi:10.1007/BF02055410
Frisch MJ et al (2004) Gaussian 03, revision B.05. Gaussian, Inc., Wallingford CT
Preston RS, Hanna S, Heberle J (1962) Phys Rev 128:2207. doi:10.1103/PhysRev.128.2207
http://orgs.unca.edu/medc/Resources-isotopes/Resource-Fe.html
Cornell RM, Schwertmann U (2003) The iron oxides. Wiley VCH, Weinheim
Vértes A, Nagy DL (eds) (1990) Mössbauer spectroscopy of frozen solutions. Akadémiai Kiadó, Budapest
Viefhaus T, Schwarz K, Hübler K, Locke K, Weidlein JZ (2001) Anorg Allg Chem 627:715. doi:10.1002/1521-3749(200104)627:4<715::AID-ZAAC715>3.0.CO;2-0
Shenoy GK, Wagner FE (eds) (1978) Mössbauer isomer shifts. Amsterdam, North Holland
Burger K, Korecz L, Manuaba IBA, Mag P (1966) J Inorg Nucl Chem 28:1673. doi:10.1016/0022-1902(66)80068-4
Danon J (1968) Lectures on the Mössbauer effect. Gordon and Breach, New York
Greenwood NN, Gibb TC (1971) Mössbauer spectroscopy. Chapman and Hall Ltd, London
Dickson DPE, Berry FJ (eds) (1986) Mössbauer spectroscopy. Cambridge University Press, Cambridge
Dufek P, Blaha P, Schwarz K (1995) Phys Rev Lett 75:3545. doi:10.1103/PhysRevLett.75.3545
Acknowledgments
This study was supported by the Hungarian Science Foundation (OTKA K068135 and K67835 and K62691). Support from the Czech-Hungarian Intergovernmental Fund, Grant No. CZ-11/2007. is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuzmann, E., Szalay, R., Vértes, A. et al. Observation and interpretation of 157.5 T internal magnetic field in Fe[C(SiMe3)3]2 coordination compound. Struct Chem 20, 453–460 (2009). https://doi.org/10.1007/s11224-009-9440-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9440-1