Abstract
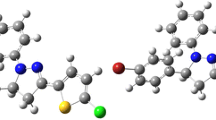
Two 2-pyrazoline derivatives of 1-phenyl-3-(4-chlorophenyl)-5-(2-chlorophenyl)-2-pyrazoline (1) and 1-phenyl-3-(4-methylphenyl)-5-(2-chlorophenyl)-2-pyrazoline (2) have been synthesized and characterized by elemental analysis, IR, UV–Vis, and fluorescence spectra. The crystal structure of 2 has been determined by X-ray single crystal diffraction. For the two compounds, density functional theory (DFT) calculations of the structures and natural population atomic charge analysis have been performed at B3LYP/6-311G** level of theory. By using TD-DFT method, electron spectra of 1 and 2 have been predicted, which are in good agreement with the experimental ones. Comparative studies on 1 and 2 indicate that the change of substituted groups in 3-phenyl ring of pyrazoline ring will change the peak intensity and peak locations both in electron spectra and fluorescence spectra.







Similar content being viewed by others
References
Tang CW, VanSlyke SA (1987) Appl Phys Lett 51:913. doi:10.1063/1.98799
Grüner J, Hamer PJ, Friend RH, Huber HJ, Scherf U, Holmes AB (1994) Adv Mater 6:748. doi:10.1002/adma.19940061006
Tasch S, Niko S, Leising G, Scherf U (1996) Appl Phys Lett 68:1090. doi:10.1063/1.115722
Greenham NC, Moratti SC, Bradly DDC, Friend RH, Holmes AB (1993) Nature 365:628. doi:10.1038/365628a0
Greenham NC, Samuel IDW, Hayes GR, Phillips RT, Kessener YARR, Moratti SC, Holmes AB, Friend RH (1995) Chem Phys Lett 241:89. doi:10.1016/0009-2614(95)00584-Q
Salbeck J, Yu N, Bauer J, Weissortel F, Bestgen H (1997) Synth Met 91:209. doi:10.1016/S0379-6779(98)80033-7
Grice AW, Tajbakhsh A, Burn PL, Bradley DDC (1997) Adv Mater 9:1174. doi:10.1002/adma.19970091511
Gao ZQ, Lee CS, Bello I, Lee ST, Chen RM, Lu TY (1999) Appl Phys Lett 74:865. doi:10.1063/1.123392
Rivett DE, Rosevear J, Wilshire JFK (1983) Aust J Chem 36:1649
Wagner A, Schellhammer CW, Petersen S (1966) Angew Chem Int Ed Engl 5:699. doi:10.1002/anie.196606991
Dorlars H, Schellhammer CW, Schroeder J (1975) Angew Chem Int Ed Engl 14:665. doi:10.1002/anie.197506651
Sarkar AK (1971) Fluorescent whitening agents. Merrow, Watford
Sano T, Fujii T, Nishio Y, Hamada Y, Shibata K, Kuroki K (1995) Jpn J Appl Phys 34:3124. doi:10.1143/JJAP.34.3124
Ji SJ, Shi HB (2006) Dyes Pigments 70:246. doi:10.1016/j.dyepig.2005.03.007
Yang GB, Wu Y, Tian WJ, Zhou X, Ren AM (2005) Curr Appl Phys 5:327. doi:10.1016/j.cap.2003.11.093
Lu ZY, Jiang Q, Zhu WG, Xie MG, Hou YB, Chen XH, Wang ZJ, Zou DC, Tsutsui T (2000) Synth Met 111–112:425. doi:10.1016/S0379-6779(99)00388-4
Wang ML, Zhang JX, Liu JZ, Xu CX, Ju HX (2002) J Lumin 99:79. doi:10.1016/S0022-2313(01)00204-6
Zhao PS, Li YF, Guo HM, Jian FF, Wang X (2007) Bull Kor Chem Soc 28:1539
Zhao PS, Li YF, Guo HM, Wang X, Jian FF (2007) Pol J Chem 81:1735
Jian FF, Zhao PS, Guo HM, Li YF (2008) Spectrochim Acta [A] 69:647. doi:10.1016/j.saa.2007.05.016
Zhao PS, Wang HY, Li RQ, Guo HM (2008) Indian J Chem A 47:986
Guo HM, Jian FF, Zhao PS, Zhang YC, Li YF (2007) Acta Crystallogr E63:o215
Sheldrick GM (1997) SHELXTL, v5 reference manual. Siemens Analytical X-Ray Systems, Madison, WI
Wilson AJ (1992) International table for X-ray crystallography, vol C: Tables 6.1.1.4 (pp 500–502) and 4.2.6.8 (pp 219–222), respectively. Kluwer Academic, Dordrecht
Dewar MJS, Zoebisch EG, Healy EF (1985) J Am Chem Soc 107:3902. doi:10.1021/ja00299a024
Peng C, Ayala PY, Schlegel HB, Frisch MJ (1996) J Comput Chem 17:49
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA, Vreven T Jr, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (2004) Gaussian, Inc., Wallingford, CT
Runge E, Gross EKU (1984) Phys Rev Lett 52:997. doi:10.1103/PhysRevLett.52.997
Petersilka M, Gossmann UJ, Gross EKU (1966) Phys Rev Lett 76:1212. doi:10.1103/PhysRevLett.76.1212
Bauernschmitt R, Ahlrichs R (1996) Chem Phys Lett 256:1996. doi:10.1016/0009-2614(96)00440-X
Jamorski C, Casida ME, Salahub DR (1996) J Chem Phys 104:5134. doi:10.1063/1.471140
Acknowledgments
This work was supported by Huaian Science & Technology Bureau, Jiangsu Province, P. R. China (HAG07025) and Fund of Huanyin Teachers College (07HSBS004, 08HSJSK003). Fund of Jiangsu Key Laboratory for Chemistry of Low-Dimensional Materials (JSKC08047).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhao, P.S., Li, R.Q., Sun, X.J. et al. Comparative study on two 2-pyrazoline derivatives with experimental and theoretical methods. Struct Chem 20, 443–451 (2009). https://doi.org/10.1007/s11224-009-9436-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-009-9436-x