Abstract
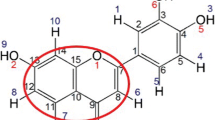
Crenulatin (C25H20O10) is a flavonol derivative and has been isolated from the roots of Rhodiola crenulata (Hook. F. et Thoms.), a widely used medicinal herb. Magnesium and calcium cations play an important physiological role in biological systems. In this work, interactions of magnesium and calcium divalent cations with Crenulatin molecule were studied. Density functional theory (DFT) was used to determine coordination geometries and absolute metal ion affinities (MIA) for all possible stable complexes. The results show that calcium and magnesium cations are able to interact with the Crenulatin molecule through mono-, bi-, and tricoordination. B3LYP/6-31G(d) bond energies for all complexes reveal that magnesium cation has a greater affinity to Crenulatin molecule than calcium cation. The calculated value of Mg2+ cation affinity, including the zero-point vibrational energy (ZPE) and basis set superposition error (BSSE), is 231.8 kcal mol−1 for the most stable complex. Entropy (ΔS) and free energy (ΔG) variations for the metalation processes considered here have also been reported.




Similar content being viewed by others
References
Darbinyan V, Kteyan A, Panossian A (2000) Phytomedicine 7:365
Seo WG, Pae HO, Oh GS (2001) J Ethnopharmacol 76:119
Pae HO, Seo WG, Oh GS (2001) Immunopharmacol Immunotoxicol 23:25
Xu KJ, Zhang SF, Lu GZ (1999) Med J NDFNC 20:172
Hao LM, Jiang WH, Meng XT (2000) Chin J Gerontol 20:230
Li Y, Cui L, Pan L (2001) Chin J Gerontol 21:55
Guan GM, Wang YP, Dong Z (1999) Chin J Otorhinolaryngol 34:227
Ma Y, Zhang XZ, Chen XS (2001) Chin Ment Health J 15:117
Chi AQ, Zhang XS, Lu XM (2000) Chin Tradit Herb Drugs 31:442
Du M, Xie JM (1994) Acta Chimi Sini 52:927
Su XF, Zhang H, Shao JX, Wu HY (2007) J Mol Struct (THEOCHEM) 847:59
Belrhali H, Yaremchuk A, Tukalo M, Berthetcolominas C, Rasmussen B, Bosecke P, Diat O, Cusack S (1995) Structure 3:341
Arnez JG, Moras D (1997) Trends Biochem Sci 22:211
Desogus G, Todone F, Brick P, Onesti S (2000) Biochemistry 39:8418
Torres-Larios A, Sankaranarayanan R, Rees B, Dock-Bregeon AC, Moras D (2003) J Mol Biol 331:201
Airas RK (1996) Eur J Biochem 240:223
Zurek J, Bowman AL, Sokalski WA, Mulholland AJ (2004) Struct Chem 15:405
Frick DN, Banik S, Rypma RS (2007) J Mol Biol 365:1017
Falcke M (2003) New J Phys 5:961
Berridge MJ, Bootman MD, Lipp P (1998) Nature 395:45
Trofimova MS, Andreev IM, Kuznetsov VV (1999) Physiol Plant 105:67
Liang H, Yuan QP, Xiao Q (2006) J Mol Catal B-Enzym 43:9
Russo N, Toscano M, Grand A (2003) J Phys Chem A 107:1533
Russo N, Toscano M, Grand A (2001) J Am Chem Soc 123:10272
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Montgomery JA Jr, Vreven T, Kudin KN, Burant JC, Millam JM, Iyengar SS, Tomasi J, Barone V, Mennucci B, Cossi M, Scalmani G, Rega N, Petersson GA, Nakatsuji H, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Klene M, Li X, Knox JE, Hratchian HP, Cross JB, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW,Ayala PY, Morokuma K, Voth GA, Salvador P, Dannenberg JJ, Zakrzewski VG, Dapprich S, Daniels AD, Strain MC, Farkas O, Malick DK, Rabuck AD, Raghavachari K, Foresman JB, Ortiz JV, Cui Q, Baboul AG, Clifford S, Cioslowski J, Stefanov BB, Liu G, Liashenko A, Piskorz P, Komaromi I, Martin RL, Fox DJ, Keith T, Al-Laham MA, Peng CY, Nanayakkara A, Challacombe M, Gill PMW, Johnson B, Chen W, Wong MW, Gonzalez C, Pople JA (1998) Gaussian 98, Revision A.9, Gaussian, Inc. Pittsburgh, PA
Becke ADJ (1993) Chem Phys 98:648
Lee C, Yang W, Parr RG (1988) Phys Rev B 37:85
Remko M, Rode BM (2004) Struct Chem 15:23
Russo N, Toscano M, Grand A (2003) J Mass Spectrom 38:65
Frisch MJ, Del Bene JE, Binkley JS, Schaefer HF (1986) J Chem Phys 84:279
Eller K, Schwarz H (1991) Chem Rev 91:121
Fontijn A (ed) (1992) Gas-phase metal reactions. North-Holland, Amsterdam
Freiser BS (ed) (1995) Organometallic ion chemistry. Kluwer Academic Publishers, Dordrecht
Cerda BA, Wesdemiotis C (1996) J Am Chem Soc 118:1884
Armentrout PB (2000) J Am Soc Mass Spectrom 11:71
Wilson MA, Brunger AT (2000) J Mol Biol 301:1237
Acknowledgments
This project was supported by the National Natural Science Foundation of China and China Academia Engineering Physics under Grant No. 10676025.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material. The optimized geometry parameters, including bond lengths, bond angles and dihedrals, of Crenulatin-a, Crenulatin-b, and their complexes at B3LYP/6-31G(d) level (Table S1–S20).
Rights and permissions
About this article
Cite this article
Cheng, X., Su, X., Zhao, X. et al. Density functional theoretical study on attachment sites of Mg2+ and Ca2+ and metal ion affinity to Crenulatin molecule. Struct Chem 19, 541–548 (2008). https://doi.org/10.1007/s11224-008-9315-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-008-9315-x