Abstract
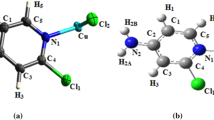
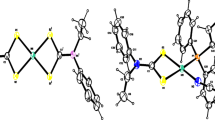
The crystal structure of 3-azido-1,2,4-triazole (AZT) has been determined. A novel coordination compound [Cd(AZT)4(H2O)2](PA)2 · 4H2O has been synthesized by using 3-azido-1,2,4-triazole as ligand, and its structure has been characterized by using X-ray single crystal diffraction, elemental analysis, and FT-IR spectroscopy. Each cadmium (II) center is coordinated with four N atoms of four AZT molecules and two O atoms of two H2O molecules to form a slightly distorted octahedron. The optimized molecular structure and NBO charges of 3-azido-1,2,4-triazole have been obtained from the density functional theory (DFT) with the B3LYP method employing the 6-311 + G** basis sets. Thermal decomposition mechanism of [Cd(AZT)4(H2O)2](PA)2 · 4H2O has been predicted based on DSC, TG-DTG, and FT-IR analyses. The kinetic parameters of the first exothermic process of [Cd(AZT)4(H2O)2](PA)2 · 4H2O were studied by applying the Kissinger’s and Ozawa-Doyle’s methods.
Index Abstract
A novel coordination compound [Cd(AZT)4(H2O)2](PA)2 · 4H2O has been synthesized by using 3-azido-1,2,4-triazole as ligand and its structure has been characterized by using X-ray single crystal diffraction, elemental analysis, and FT-IR spectroscopy. Each cadmium (II) center is coordinated to form a slightly distorted octahedron. Thermal decomposition mechanism of [Cd(AZT)4(H2O)2](PA)2 · 4H2O has been predicted based on DSC, TG-DTG, and FT-IR analyses.







Similar content being viewed by others
References
Zhai QG, Wu XY, Chen SM, Lu CZ, Yang WB (2006) Cryst Growth Design 6:2127
Haasnoot JG (2000) Coordin Chem Rev 200–202:131
Shiu KB, Guo WN, Peng SM, Cheng MC (1994) Inorg Chem 33:3010
Romero MA, Salas JM, Quiros M, Sanchez MP, Molina J, Bahraoui JE, Faure R (1995) J Mol Struct 345:189
Haasnoot JG, Driessen WL, Reedijk J (1984) Inorg Chem 23:2803
Dillen J, Lenstra ATH, Haasnoot JG, Reedijk J (1983) Polyhedron 2:195
Pei Y, Lang A, Bergerat P, Kahn O, Fettouhi M, Ouahab L (1996) Inorg Chem 35:193
Kunkeler PJ, Van Koningsbruggen PJ, Cornelissen JP, Van der Horst AN, Van der Kraan AM, Spek AL, Haasnoot JG, Reedijk J (1996) J Am Chem Soc 118:2190
Janiak C, Scharmann TG, Bräuniger T, Holubová J, Nadvornik M (1998) Z Anorg Allg Chem 624:769
Vreugdenhil W, Haasnoot JG, Kahn O, Thuéry P, Reedijk J (1987) J Am Chem Soc 109:5272
Trofimenko S (1972) Chem Rev 72:497
Lynch M, Hyde KE, Bocko PL, Kokoszka GF (1977) Inorg Chem 16:562
Inoue M, Emori S, Kubo M (1968) Inorg Chem 7:1427
Inoue M, Kishita M, Kubo M (1965) Inorg Chem 4:626
Hage R, Haasnoot JG, Nieuwenhuis HA, Reedijk J, de Ridder DJA, Vos JG (1990) J Am Chem Soc 112:9245
Kröber J, Codjovi E, Kahn O, Grolière F, Jay C (1993) J Am Chem Soc 115:9810
Janiak C, Scharmann TG, Bräuniger T, Holubová J, Nádvorník M (1998) Z Anorg Allg Chem 624:769
Yi L, Ding B, Zhao B, Cheng P, Liao DZ, Yan SP, Jiang ZH (2004) Inorg Chem 43:33
Du JY (2004) Transit Metal Chem 29:699
Bichay M, Fronabarger JW, Gilardi R, Butcher RJ, Sanborn WB, Sitzmanna ME, Williams MD (2006) Tetrahedron Lett 47:6663
Liu JC, Guo GC, Huang JS, You XZ (2003) Inorg Chem 42:235
Zhou JH, Cheng RM, Song Y, Li YZ, Yu Z, Chen XT, Xue ZL, You XZ (2005) Inorg Chem 44:8011
Ugryumov IA, Ilyushin MA, Tselinskii IV, Kozlov AS (2003) Russ J Appl Chem 76:439
Chernai AV, Sobolev VV, Chernai VA, Ilyushin MA, Dlugashek A (2003) Combust Explo Shock Waves 39:335
Zhang JG, Zhang TL, Liu YH (2005) Chinese J Chem 23:1403
Ma GX, Zhang TL, Zhang JG, Yu KB (2004) Z Anorg Allg Chem 630:423
Zhang JG, Zhang TL, Yang L, Mao LQ (2002) Chinese J Inorg Chem 18:284
Zhang JG, Zhang TL, Lu Z, Yu KB (1999) Acta Chim Sinica 57:1233
Zhang TL, Hu RZ, Li FP, Yu KB (1994) Acta Chim Sinica 52:545
Zhang JG, Zhang TL (2000) Acta Chim Sinica 58:1563
Zhang JG, Zhang TL (2000) Acta Phys-Chim Sinica 16:1110
Zhang TL, Zhang JG, Zhang ZG (2000) Acta Chim Sinica 58:533
Hammerl A, Klapötke TM, Nöth H, Warchhold M, Holl G (2003) Propellants Explos Pyrotech 28:165
Hammerl A, Klapötke TM, Mayer P, Weigand JJ, Holl G (2005) Propellants Explos Pyrotech 30:17
Xue H, Shreeve JM (2005) Adv Mater 17:2142
Denault GC, Marx PC, Takimoto HH (1968) J Chem Eng Data 13:514
Xue H, Gao Y, Twamley B, Shreeve JM (2005) Chem Mater 17:191
Xue H, Gao Y, Twamley B, Shreeve JM (2005) Inorg Chem 44:5068
Kofman TP, Krasnov KN (2004) Russ J Org Chem 40:1651
Sheldrick GM (1997) SHELXS-97. University of Göttingen, Germany
Sheldrick GM (1997) SHELXL-97. University of Göttingen, Germany
Becke A (1993) J Chem Phys 98:5648
Lee C, Wang W, Parr RG (1988) Phys Rev B37:785
Reed AE, Weinstock RB, Weinhold F (1985) J Chem Phys 83:735
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE (1998) GAUSSIAN 98, revision A7. Gaussian, Inc., Pittsburgh PA
Tarver CM, Goodale TC, Cowperthwaite M, Hill ME (1977) AD-A044714
Zhang JG, Zhang TL, Yu KB (2001) Acta Chim Sinica 59:84
Zhang JG, Zhang TL, Yang L, Mao LQ (2002) Chinese J Inorg Chem 18:284
Acknowledgments
This work is supported by NSAF Foundation (No. 10776002) of National Natural Science Foundation of China and China Academy of Engineering Physics.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cui, Y., Zhang, T.L., Zhang, J.G. et al. Preparation, molecular structure, and thermal analyses of a novel coordination compound [Cd(AZT)4(H2O)2](PA)2 · 4H2O (AZT = 3-azido-1,2,4-triazole, PA = picrate). Struct Chem 19, 269–278 (2008). https://doi.org/10.1007/s11224-008-9282-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11224-008-9282-2